Consolidated Financial Results for the Fiscal Year Ending March 31, 2013
|
|
|
- Brent Shields
- 5 years ago
- Views:
Transcription
1 Consolidated Financial Results for the Fiscal Year Ending March 31, Outline of Consolidated Financial Results 2. Highlights of Business Performance 3. Consolidated Financial Results 4. Main Product Sales Update 5. Actual and Forecast of Main Subsidiary Companies 6. R&D Expenses, Capex & Depreciation 7. Main R&D Activities reference 8. Segment information 9. P&L Summary 10. BS Summary 11. Financial summary 12. KYORIN Pharmaceutical result P.1 P.2 P.3 P.4 P.5 P.6 P.7 8 P.10 P11 12 P P.13 P.14 P May 9, 2013 KYORIN Holdings, Inc. These forecast performance figures are based on information currently available to the Company and may include uncertain factors or risk that affect our future performance. Accordingly, actual business results may materially differ from the forecasted figures due to various factors in the future.
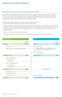
2 Outline of Consolidated Financial Results for Fiscal 2012 ( million) FY2009 FY2010 FY2011 FY2012 change FY2013 (forecast) change Net sales 99, , , , % 113, % Operating income 13,261 16,443 14,464 17, % 18, % Ordinary income 14,234 17,110 15,275 18, % 19, % Net income 8,848 10,927 9,231 12, % 12, % Net income per share (yen) % % Total assets 137, , , , % Total equity 104, , , , % Consolidated Business Results for Fiscal 2012 (Actual) [Net Sales] Sales of ethical drug and generic drug increased year on year. And sales of KYORIN Pharmaceutical Facilities which commenced operations on Oct 1 st /2012 contributed to increase of net sales. As a result, consolidated net sales increased 3.7% year on year, to 107.0bln for the maximum sales in the past. [Income] While cost of sales ratio increased 1.7% due to the drug price revision, effect of KYORIN pharmaceutical Facilities sales, gross profitroserose 0.6bln due to the increase of net sales. SG & A expenses fell given a decrease in R&D expenses(-20.8% YoY). Consequently, operating income rose 24.1% to 17.9bln, net income rose 34.6% to 12.4bln year on year, for the maximum in the past. Consolidated Business Results Forecast for Fiscal 2013 [Net Sales and Income] While SG & A expenses increase, we expect sales to grow due to ethical drug sales in Japan and generic drugs sales, and effect of KYORIN pharmaceutical Facilities full year sales. As a result, we expect net sales to increase 5.6% year on year to 113.0bln, operating income to increase 3.6% to 18.6bln, and net income to increase 2.2% to 12.7bln. 1
3 Highlights of Business Performance Units: billion Net sales Cost of Sales 36.9 Cost of Sales Ratio 35.8% R&D 14.0 SG&A (excluding R&D) 37.9 SG&A 51.8 Operating Income Net sales Cost of Sales 40.1 Cost of Sales Ratio 37.5% R&D 11.1 SG&A (excluding R&D) SG&A 48.9 Operating Income Highlight Net Sales increased 3.8bln year on year. increase of Japan ethical and generic drugs KYORIN Pharmaceutical Facilities commenced operations on Oct 1 st /2012 Highlight g Cost of Sales Ratio increased 1.7points. Sales of generic drugs increased. Drug price revision (in the 6% range for KYORIN pharmaceutical) Effect of KYORIN pharmaceutical facilities sales. Gross profit increased 0.6bln year on year. Highlight SG&A Expenses decreased 2.9bln year on year. (mainly, decrease of R&D Expenses) Operating Income increased 3.4bln year on year. FY11 FY12 2
4 Consolidated Financial Results : vs FY2011 billion FY2011 FY2012 change Net Sales (total) Ethical drugs Business Sales of new 88.0 ethical drugs Japan Overseas Generic drugs Over-thecounter drugs Healthcare (Skin care) Business Operating Income Ordinary Income Net Income change Net Sales 107.0bln (+ 3.8) Ethical drugs business 105.2bln (+ 4.5) Sales of new ethical drugs 88.3bln (+ 2.3) FY2011 FY2012 Kipres Mucodyne Pentasa Uritos (+ 2.8) ( 2.4) ( 0.4) ( + 1.2) Sales of new ethical drugs in Overseas 2.4bln (+ 0.4) Gatifloxacin ( 0.1) Sales of Generic drugs 10.1bln (+ 1.4) Health insurance pharmacy market s sales and contract manufacturing s sales increased. Sales of Over-the-counter drugs 4.4bln (+ 0.4) increase of Milton and over the counter drugs Healthcare (Skin care) Business 1.9bln ( 0.7) Sales declined at Dr. Program ( 0.7) Operating Income 17.9bln (+ 3.4) Operating Income margin increased 2.8 percentage points to 16.8% Cost of Sales Ratio : UP 1.7 percentage points (35.8% 37.5%) Sales of generic drugs increased. Drug gp price revision (in the 6% range for KYORIN pharma) Effect of KYORIN pharmaceutical facilities sales. R&D Ratio : DOWN 3.2 percentage points (13.5% 10.3%) 14.0bln 11.1bln (DOWN 2.9bln) Expenses associated with the progress of the R&D were posted in the previous year. (KRP-108Ph3 PENTASA Ph3 discontinued of KRP-104) SG&A Ratio (excluding R&D expenses) : DOWN 1.3 percentage points 36.7% 35.4% 37.9bln 37.9bln Net Income 12.4bln (+ 3.2) Dividend per share(interim dividend 10.0)
5 Main Product Sales Update Units: billion Sales of new ethical drugs (Japan) FY2008 FY2009 FY2010 FY2011 actual FY2012 change FY2013 (forecast) Kipres LT receptor antagonist % 40.9 Mucodyne Mucoregulant % 18.7 Pentasa (Ulcerative colitis and Crohn s disease treatment) % 18.6 Uritos Kyorin (Overactive bladder) % 8.4 Ketas (For bronchial asthma and cerebrovasculas disorders) % 2.7 Sales of new ethical drugs (over seas) Gatifloxacin (Bulk Royalty) % 2% % 21 Over-the- Milton counter drugs (Disinfectant) % 2.1 4
6 Actual and Forecast of Main Subsidiary Companies KYORIN pharmaceutical FY2011 FY2012 FY2013(forecast) Sales Operating Income Net Income Units: billion KYORIN Rimedio FY2011 FY2012 FY2013(forecast) Sales Operating Income Net Income Dr. Program FY2011 FY2012 FY2013(forecast) Sales Operating Income Net Income
7 R&D Expenses, Capex & Depreciation <Consolidated> FY2008 FY2009 FY2010 FY2011 actual FY2012 change Units: million FY2013 forecast R&D expenses 10,531 11,807 12,495 13,964 11, % 12,100 Capital expenditure 1,612 1,291 1,668 1,952 6, % % 4,400 Depreciation expense 3,799 2,810 2,458 2,363 2, % 3,300 <Capital expenditure (Actual/Forecast)> FY2011 FY2012 Units: billion FY2013 forecast Plant facilities Equipment for control, sales activities Equipment for research
8 Main R&D Activities -1 (May 5, 2013 Release) Ph III ~ Application submitted Changes from the previous announcement (Feb 4, 2013) Japan Stage Overseas Compound/ Code Therapy area/action Oi Origini Features Comments Approved (3/2013) Pentasa (suppository) Ulcerative colitis Ferring Pharmaceuticals Consideration of a new dosage form for the active phase of ulcerative colitis (once a day) Development of a new dosage form Ph completed(2/2012) Application submitted (9/2012) (US) SkyePharma : Application submitted (3/2009) Europe Mundipharma :Launched(9/2012) KRP-108 (Inhalant) Antiasthmatic SkyePharma PLC An ICS/LABA combination product, which offers better compliance and convenience to the patients License agreement with SkyePharma (4/2008) Domestic Ph II completed (4/2010) Ph completed(3/2012) Ph 9/2012 Europe Almirall : Launched(9/2012) (US) Forest Pharmaceuticals : Launched (12/2012) KRP-AB1102 Inhaled drug Chronic Obstructive Pulmonary Disease (COPD) Almirall - New Chemical Entity: Aclidinium Bromide - Long Acting Muscarinic Agonist (LAMA) - Twice Daily administration -Onset of Action on the first day Genuair 1) Designed with a feedback system, which through a 'colored control window' and an audible click helps confirm that the patient has inhaled correctly 2) Counter for remaining doses 3) Safety features such as an anti-double-dosing dosing mechanism and an end-of-dose lock-out system to prevent use of an empty inhaler License agreement with Almirall (2/2011) Ph (4/2013) KIPRES Chewable Oral Granules Bronchial Asthma Merck For pediatric patient Allergic Rhinitis Additional indication Co-development with MSD K.K. 7
9 Main R&D Activities -2 (May 5, 2013 Release) POC Project (Pre-clinical ~ Ph II) Changes from the previous announcement (Feb 4, 2013) Japan Stage Overseas Compound/ Code Therapy area/action Origin Features Comments Ph 5/2012 (Europe & US) Almirall: Ph (US) Forest Laboratories : Ph KRP-AB1102F (Fixed dose combination inhaled drug) Chronic Obstructive Pulmonary Disease (COPD) Almirall Combination of aclidinium bromide with the long acting beta agonist formoterol : This combination is aimed at providing higher efficacy than each component alone,as well as the improved convenience of having the two products in the same easy to use inhalation device.this is currently in phase clinical development. Ph 8/2011 Ph Merz KRP-209 Tinnitus Merz KRP-209 (Neramexane) is expected to improve the patients' annoyance and difficulties in their life caused by tinnitus, mainly through its two pharmacological properties: 1) NMDA antagonistic activity and 2) Nicotinic acetylcholine antagonistic activity License agreement with Merz (11/2009) Merz:Ph I clinical trial of Japanese patients in US completed (3/2010) Ph 3/2013 Ph POC) (12/2010) (Novartis) KRP-203 Transplantation, autoimmune diseases,andand IBD In-house An immunosuppressant with a novel mechanism called an S1P-agonist. It may have a better safety profile than previous ones as well as an excellent effect under concomitant use with other types of immunomodulator. License agreement with Novartis (2/2006) New license agreement IBD (11/2010) Ph 8/2011 Ph 7/2012 KRP-AM1977X (Oral agent KRP-AM1977Y (Injection New quinolone synthetic antibacterial agent New quinolone synthetic antibacterial agent In-house In-house Superior ability to combat drug-resistant grampositive bacteria (incl. MRSA) Outstanding ADME (oral absorption, tissue migration) High degree of safety expected since safety hurdles cleared prior to clinical trials 8
10 Reference 9
11 Segment information Sales, Profit or Loss of each report segment Units: billion Sales change Profit change Net Sales (total) Ethical drugs business Sales of new ethical drugs Japan Overseas Generic drugs Over-the-counter drugs Healthcare(Skincare) business Amount of adjustment (Note ) The Company is applying the Revised Accounting Standard for Disclosures about Segments of an Enterprise and Related Information and the Guidance on the Accounting Standard for Disclosures about Segments of an Enterprise and Related Information. As a result, the reported segments are the Ethical Drugs Business and the Consumer Healthcare Business. 10
12 P&L Summary: Consolidated Results (1) Units: million FY2011 FY2012 Actual % Sales Actual % Sales % Change Change Sales 103, % 107, % 3.7% 3,798 Ethical Drugs business Sales of new Ethical Drugs 100, % 105, % 4.5% 4,507 88, % 90, % 3.0% 2,675 Japan 85, % 88, % 2.7% 2,290 Overseas 2, % 2, % 19.1% 385 Generic Drugs 8, % 10, % 16.6% 1,439 Over-the- counter Drugs 3,987 39% 3.9% 4,379 41% 4.1% 9.8% 392 and Others Consumer Healthcare 2, % 1, % 27.5% 708 Business <Subsidiaries and Equity-method Affiliates> Consolidated subsidiaries (8) KYORIN Pharmaceutical Co., Ltd. Kyorin USA, Inc. Kyorin Europe GmbH ActivX Biosciences, Inc. KYORIN Rimedio Co., Ltd. Dr. Program Co., Ltd. KYORIN Medical Supply Co., Ltd. KYORIN Pharmaceutical Facilities Co., Ltd Equity-Method Affiliates Nippon Rika Co., Ltd. Breakdown change Sales 107,031 3,798 Ethical drug sales in Japan 88,286 2,290 Kipres Mucodine Pentasa Uritos FY2011 FY2012 :billion ( 2.8) ( 2.4) ( 0.4) ( 1.2) Ethical drug sales overseas Gatifloxacin 2, ( 0.1) 01) Generic Drugs 10,095 1,439 Health insurance pharmacy market s sales and contract manufacturing s sales increased. Over-the-counter Drugs and Others 4, increase of Milton and over the counter drugs Consumer Healthcare Business 1, decrease of Dr.Program s sales 11
13 P&L Summary: Consolidated Results (2) FY2011 FY2012 Units: million Breakdown Actual % Sales Actual % Sales % Change Change Sales 103, % 107, % 3.7% 3,798 Cost of Sales 36, % 40, % 8.7% 3,207 Gross Profit 66, % 66, % 0.9% 591 SG&A (Incl. R&D expenses) Operating Income Non-Operating Income Non-Operating Expenses 51,842 (13,964) 50.2% 13.5% 48,949 (11,059) 45.7% 10.3% 5.6% 20.8% 2,892 2,904 14, % 17, % 24.1% 3, % 0.1% % 0.1% 10.1% 8.5% 88 5 Ordinary Income 15, % 18, % 22.3% 3,401 Extraordinary Profits Extraordinary Losses Income before income taxes Corporate, inhabitants and enterprise taxes % 0.0% % 0.1% 15.4% 182.0% , % 18, % 21.9% 3,340 5, % 5, % 13.3% 689 Tax adjustments % 0.8% % 0.3% 63.3% 3% 539 Net Income 9, % 12, % 34.6% 3,190 Cost of Sales Ratio : UP 1.7 percentage points ( ) Sales of generic drugs increased. Drug price revision (in the 6% range for KYORIN pharma) Effect of KYORIN pharmaceutical facilities sales R&D Ratio : DOWN 3.2 percentage Points ( ) 14.0bln 11.1bln(decrease 2.9bln) Expenses associated with the progress of the R&D were posted in the previous year. (KRP-108Ph3 PENTASA Ph3 discontinued of KRP-104) SG&A (exclude R&D) Expenses : DOWN 1.3 percentage Points 37.9bln 37.9bln Operating Income ( ) 17,948( 3,484) Operating Income margin increased 2.8percentage points to 16.8% Net Income 12,422( 3,190) Dividend per share Consolidated payout ratio 30.1% 12
14 BS Summary: Consolidated Results Units: million FY2011 FY2012 Actual %total Actual % total change Breakdown Current Assets 99, % 108, % 9% 8,414 Cash, deposits Notes and accounts receivable Mk securities Inventory Other 21,615 45,067 7,372 20, ,370 46,555 11,667 19, Other 5,056 8,733 Fixed Assets 45, % 46, % 880 Tangible assets Intangible assets Investments 14,544 18, ,431 27,577 Total Assets 145, % 154, % 9,295 Current Liabilities 23, % 22, % 487 Notes payable Other 9,043 14,341 8,556 14,341 Non-Current Liabilities 4, % 2, % 1,115 Total Liabilities 27, % 25, % 1,603 Owner s Equity 117, % 126, % 9,054 Other Comprehensive Income % 2, % 1,843 Unrealized holding gain (loss) on securities 537 2,293 Foreign currency translation adjustments Total Equity 118, % 129, % 10,898 Current tasset 8,414mil Cash, deposits 245mil Notes and accounts receivable 1,487mil Mk securities 4,295mil Inventory 800mil Other 3,677mil Fixed Assets 880mil Tangible Assets 3,665mil Intangible Assets 70mil Investments 2,854mil Current Liabilities 487mil Notes Payable able 486mil Other 0mil Non-Current Liabilities 1,115mil Total Liabilities and Equity 145, % 154, % 9,295 13
15 Financial summary (Consolidated) million FY2008 FY2009 FY2010 FY2011 FY2012 FY2013 (forecast) Sales 90,889 99, , , , ,000 (Exports) (3,830) (2,693) (2,784) (2,059) (2,400) (2,100) Cost of Sales cost of Sales Ratio SG&A R&D Expenses 36,791 (40.5%) 45,146 (49.7%) 37,477 (37.6%) 49,025 (49.1%) 37,554 (36.1%) 50,071 (48.1%) 36,926 (35.8%) 51,842 (50.2%) 40,133 (37.5%) 48,949 (45.7%) (49.7%) (49.1%) (48.1%) (50.2%) (45.7%) Operating Income Ordinary Income Net Income 10,531 (11.6%) 8,952 (9.8%) 9,208 (10.1%) 2,037 (2.2%) 11,807 (11.8%) 13,261 (13.3%) 14,234 (14.3%) 8,848 (8.9%) 12,495 (12.0%) 16,443 (15.8%) 17,110 (16.4%) 10,927 (10.5%) 13,964 (13.5%) 14,464 (14.0%) 15,275 (14.8%) 9,231 (8.9%) 11,059 (10.3%) 17,948 (16.8%) 18,676 (17.4%) 12,422 (11.6%) 12,100 (10.7%) 18,600 (16.5%) 19,200 (17.0%) 12,700 (11.2%) EPS ( ) Capital Assets 124, , , , ,968 Total Equity 96, , , , ,985 BPS ( ) 1, , , , ,099 ROE (%) 2.1% 8.8% 10.1% 8.0% 1, Equity Ratio (%) 77.5% 76.5% 75.9% 81.1% 10.0% Employees 2,247 2,246 2,294 2, % Capital Expenditure 1,612 1,291 1,668 1,952 2,444 Depreciation Expense 3,799 2,810 2,458 2,363 6,576 4,400 14
16 P&L summary : KYORIN pharmaceutical (Non-consolidated)-(1) FY2011 FY2012 Units: million Breakdown Sales 95,894mil 2,197mil Ethical drug sales in Japan Actual % Sales Actual % Sales % Change Change Sales 93, % 95, % 2.3% 2,197 86,698mil FY mil FY2012 ( billion) Ethical Drugs business Sales of new Ethical Drugs 93, % 95, % 2.3% 2,197 87, % 88, % 1.2% 1,075, Kipres Mucodyne Pentasa Uritos Japan 85, % 86, % 0.8% 702 Overseas 1, % 2, % 19.6% 373 Generic Drugs 3, % 4, % 28.4% 909 Over-thecounter Drugs and Others 2, % 2, % 8.2% 211 Ethical drug sales overseas 2,277mil 373mil Gatifloxacin Generic Drugs 4,116mil 909mil Mainly the effect from consolidating distribution at KYORIN Rimedio Over-the-counter Drugs and Others 2,802mil 211mil milton
17 P&L summary : KYORIN pharmaceutical (Non-consolidated)-(2) FY2011 Actual % Sales Actual % Sales FY2012 % Change Units: million Change Sales 93, % 95, % 2.3% 2,197 Cost of Sales 32, % 33, % 5.7% 1,822 Gross Profit 61, % 62, % 0.6% 374 SG&A (R&D Expenses) 47,679 (13,472) 50.9% (14.4%) 44,898 (10,733) 46.8% (11.2%) 5.8% 20.3% 2,780 2,739 Operating Income 13, % 17, % 22.6% 3,155 Non-Operating Income Non-operating Expenses 1, % 0.0% 1, % 0.0% 6.7% 22.1% 79 5 Ordinary Income 15, % 18, % 20.4% 3,082 Extraordinary Profits Extraordinary Losses Income before Income taxes Corporate, inhabitants and enterprise taxes % 0.0% % 0.1% 14.2% 260.2% , % 1% 18, % 20.0% 0% 3,026 5, % 5, % 10.5% 534 Tax adjustments % % 22.1% 165 Net Income 9, % 11, % 28.7% 2,657 Breakdown Cost of Sales Ratio : UP 1.1 percentage points ( ) Reason for increase : Sales of generic drugs increased. Drug price revision(in the 6% range for KYORIN pharmaceutical) R&D Ratio : DOWN 3.2 percentage Points ( ) 13.5bln 10.7bln(decrease of approx. 2.8bln) Reason for decrease : Expenses associated with the progress of the R&D were posted in the previous year. (KRP-108Ph3 PENTASA Ph3 discontinued of KRP-104) SG&A (exclude R&D) Expenses : DOWN 0.9 percentage Points Operating Income ( ) 17,127 ( 3,155) Operating Income margin increased 3.0percentage points to 17.9% Net Income 11,931 ( 2,657) 16
18 BS Summary: KYORIN Pharmaceutical (Non-consolidated) FY2011 FY2012 Units: million Actual % total Actual % total change Current Assets 80, % 84, % 3,499 Cash, deposits Accounts receivable 9,444 42,046 7,192 43,320 Mk securities 7,309 11,604 Inventory 17,877 16,615 Other 3,863 5,308 Fixed Assets 41, % 37, % 3,603 Tangible assets Intangible assets Investments 11,497 11, ,558 26,215 Total Assets 121, % 121, % 103 Current Liabilities 16, % 16, % 354 Notes Payable Other 6,609 9,916 5,445 11,435 Non-Current Liabilities 3, % 2, % 1,125 Total Liabilities 19, % 19, % 770 Owner s Equity 101, % 100, % 1,065 Valuation and translation % 2, % 1,732 adjustments Total Equity 102, % 102, % 666 Breakdown Current tassets 3,499mil 3 Cash, deposits 2,252mil Accounts receivable + 1,273mil Mk securities 4,295mil Inventory 1,261mil Fixed Assets 3,603mil Tangible Assets 156mil Intangible Assets 104mil Investments 3,343mil Current Liabilities 354mil Notes Payable 1,164mil Other 1,518mil Non-Current Liabilities 1,125mil Total Liabilities and Equity 121, % 121, %
19 Financial Summary: KYORIN Pharmaceutical (Non-consolidated) million FY2008 FY2009 FY2010 FY2011 FY2012 FY2013 (forecast) Sales 77,962 85,308 92,531 93,697 95, ,000 (Exports) (3,148) (2,563) (2,642) (1,904) (2,277) 277) (1,900) Cost of Sales cost of sales ratio SG&A R&D Expenses Operating Income Ordinary Income Net Income 29,551 (37.9%) 28,374 (33.3%) 31,227 (33.7%) 32,046 (34.2%) 33,868 (35.3%) 39,894 43,795 45,658 47,679 44,898 ( ) (51.2%) (51.3%) (49.3%) (50.9%) (46.8%) 10,056 (12.9%) 8,517 (10.9%) 9,463 (12.1%) 4,041 (5.2%) 11,121 (13.0%) 13,139 (15.4%) 14,580 (17.1%) 9,472 (11.1%) 11,867 (12.8%) 15,645 (16.9%) 16,729 (18.1%) 10,732 (11.6%) 13,472 (14.4%) 13,971 (14.9%) 15,126 (16.1%) 9,274 (9.9%) 10,733 (11.2%) 17,127 (17.9%) 18,209 (19.0%) 11,931 (12.4%) 11,300 (11.3%) 17,700 (17.7%) 18,600 (18.6%) 12,400 (12.4%) EPS ( ) Capital 4,317 4,317 4,317 4,317 4,317 Assets 108, , , , ,881 Total Equity 88,470 95,505 95, , ,844 BPS ( ) 1, , , , , ROE (%) 4.6% 10.3% 11.2% 9.4% 11.6% Equity Ratio (%) 81.5% 79.7% 77.9% 83.8% 84.4% Employees poyees 1,716 1,724 1,804 1,798 1,797 Capital Expenditure 969 1,051 1,019 1,425 1,507 2,800 Depreciation Expense 3,042 2,198 1,968 1,790 1,743 1,800 18
May 11, 2017 KYORIN Holdings, Inc. P.13 P.2 P.3 P.15. reference 7. Segment information 8. P&L Summary 9. BS Summary
 Consolidated Financial Results for the Fiscal Year Ending March 31, 2017 1. Outline of Consolidated Financial Results 2. Highlights of Business Performance 3. Consolidated Financial Results 4. Main Product
Consolidated Financial Results for the Fiscal Year Ending March 31, 2017 1. Outline of Consolidated Financial Results 2. Highlights of Business Performance 3. Consolidated Financial Results 4. Main Product
Second Quarter Consolidated Financial Results for the Fiscal Year Ending March 31, 2018
 Second Quarter Consolidated Financial Results for the Fiscal Year Ending March 31, 2018 1. Outline of Consolidated Financial Results P.1 2. Highlights of Business Performance P.2 3. Consolidated Financial
Second Quarter Consolidated Financial Results for the Fiscal Year Ending March 31, 2018 1. Outline of Consolidated Financial Results P.1 2. Highlights of Business Performance P.2 3. Consolidated Financial
Interim Term Financial Results Ended September November 9,2011 KYORIN Holdings, Inc. President Masahiro Yamashita
 Interim Term Financial Results Ended September 2011 November 9,2011 KYORIN Holdings, Inc. President Masahiro Yamashita Outline of Consolidated Financial Results for the Interim Term Ended September 2011
Interim Term Financial Results Ended September 2011 November 9,2011 KYORIN Holdings, Inc. President Masahiro Yamashita Outline of Consolidated Financial Results for the Interim Term Ended September 2011
Interim Term Financial Results Ended September November 8, 2016 KYORIN Holdings, Inc. President Minoru Hogawa
 Interim Term Financial Results Ended September 2016 November 8, 2016 KYORIN Holdings, Inc. President Minoru Hogawa Outline of Consolidated Financial Results Trends of mainstay products and Generic drugs
Interim Term Financial Results Ended September 2016 November 8, 2016 KYORIN Holdings, Inc. President Minoru Hogawa Outline of Consolidated Financial Results Trends of mainstay products and Generic drugs
ANNUAL REPORT. KYORIN Holdings, Inc.
 2016 ANNUAL REPORT Year ended March 31, 2016 KYORIN Holdings, Inc. Corporate Philosophy of the Kyorin Group Kyorin continues to fulfill its mission of cherishing life and benefiting society by contributing
2016 ANNUAL REPORT Year ended March 31, 2016 KYORIN Holdings, Inc. Corporate Philosophy of the Kyorin Group Kyorin continues to fulfill its mission of cherishing life and benefiting society by contributing
2O17. ANNUAL REPORT Year ended March 31, KYORIN Holdings, Inc.
 2O17 ANNUAL REPORT Year ended March 31, 2017 KYORIN Holdings, Inc. Corporate Philosophy of the Kyorin Group Kyorin continues to fulfill its mission of cherishing life and benefiting society by contributing
2O17 ANNUAL REPORT Year ended March 31, 2017 KYORIN Holdings, Inc. Corporate Philosophy of the Kyorin Group Kyorin continues to fulfill its mission of cherishing life and benefiting society by contributing
ONO PHARMACEUTICAL CO., LTD.
 ONO PHARMACEUTICAL CO., LTD. May 13, 2009 Ono Pharmaceutical Co., Ltd. has announced its consolidated financial results for the year ended March 31, 2009. This Annual Flash Report 2009 (unaudited) is a
ONO PHARMACEUTICAL CO., LTD. May 13, 2009 Ono Pharmaceutical Co., Ltd. has announced its consolidated financial results for the year ended March 31, 2009. This Annual Flash Report 2009 (unaudited) is a
Financial Results for the Fiscal Year ended March 31, 2017 (IFRS, Consolidated)
 Financial Results for the Fiscal Year ended March 31, 2017 (IFRS, Consolidated) Company name: Mitsubishi Tanabe Pharma Corporation Stock exchange listings: Tokyo Securities code number: 4508 URL: http://www.mt-pharma.co.jp/
Financial Results for the Fiscal Year ended March 31, 2017 (IFRS, Consolidated) Company name: Mitsubishi Tanabe Pharma Corporation Stock exchange listings: Tokyo Securities code number: 4508 URL: http://www.mt-pharma.co.jp/
(April 1, September 30, 2008) October 31, 2008
 (April 1, 2008 - September 30, 2008) October 31, 2008 Net Sales Cost of Sales SG&A R&D Total Expense Operating Income Ordinary Income Net Income Results for 2Q FY2008 (vs 2Q FY2007) FY2007 1st Half Results
(April 1, 2008 - September 30, 2008) October 31, 2008 Net Sales Cost of Sales SG&A R&D Total Expense Operating Income Ordinary Income Net Income Results for 2Q FY2008 (vs 2Q FY2007) FY2007 1st Half Results
Financial Results for the 1 st Quarter of Fiscal Year Ending March 31, 2019
 Financial Results for the 1 st Quarter of Fiscal Year Ending March 31, 2019 August 8, 2018 Chiyoda Corporation Chiyoda Corporation 2018, All Rights Reserved. Index 1. Results Highlights 2 2. Financial
Financial Results for the 1 st Quarter of Fiscal Year Ending March 31, 2019 August 8, 2018 Chiyoda Corporation Chiyoda Corporation 2018, All Rights Reserved. Index 1. Results Highlights 2 2. Financial
Summary of Financial Statements for the Three Months Period Ended June 30, 2018 (IFRS, Consolidated) July 31, 2018
 Period Ended June 30, 2018 (IFRS, Consolidated) July 31, 2018 Takeda Pharmaceutical Company Limited Stock exchange listings: Tokyo, Nagoya, Fukuoka, Sapporo TSE Code: 4502 URL: http://www.takeda.com Representative:
Period Ended June 30, 2018 (IFRS, Consolidated) July 31, 2018 Takeda Pharmaceutical Company Limited Stock exchange listings: Tokyo, Nagoya, Fukuoka, Sapporo TSE Code: 4502 URL: http://www.takeda.com Representative:
Kyowa Hakko Kirin Co., Ltd.
 Kyowa Hakko Kirin Co., Ltd. Consolidated Financial Summary (IFRS) Fiscal 2018 Third Quarter (January 1, 2018 September 30, 2018) This document is an English translation of parts of the Japanese-language
Kyowa Hakko Kirin Co., Ltd. Consolidated Financial Summary (IFRS) Fiscal 2018 Third Quarter (January 1, 2018 September 30, 2018) This document is an English translation of parts of the Japanese-language
Review of Fiscal 2001
 Fujisawa Pharmaceutical Company Limited and Consolidated Subsidiaries Selected Financial Data Years Ended March 31 2001 2000 1999 1998 1997 Results for the year: Net sales... 297,517 289,142 277,281 281,584
Fujisawa Pharmaceutical Company Limited and Consolidated Subsidiaries Selected Financial Data Years Ended March 31 2001 2000 1999 1998 1997 Results for the year: Net sales... 297,517 289,142 277,281 281,584
Investor Meeting on Q2 FY2017 Results
 Investor Meeting on FY2017 Results Akira Kurokawa President & CEO November 2, 2017 Copyright 2017 Santen Pharmaceutical Co., Ltd. All rights reserved. 1 Santen s Values By focusing on ophthalmology, Santen
Investor Meeting on FY2017 Results Akira Kurokawa President & CEO November 2, 2017 Copyright 2017 Santen Pharmaceutical Co., Ltd. All rights reserved. 1 Santen s Values By focusing on ophthalmology, Santen
CMIC HOLDINGS Co., Ltd. Consolidated Financial Results
 (Note) This translation is prepared and provided for readers' convenience only. In the event of any discrepancy between this translated document and the original Japanese document, the original document
(Note) This translation is prepared and provided for readers' convenience only. In the event of any discrepancy between this translated document and the original Japanese document, the original document
(Figures are rounded down to the nearest million yen) 1. Consolidated Performance for the three months ended June 30, 2013
 [ Disclaimer: The following is meant to be an accurate translation from the original Financial Report of Santen Pharmaceutical Co., Ltd., written in ese, and is prepared for the information disclosure
[ Disclaimer: The following is meant to be an accurate translation from the original Financial Report of Santen Pharmaceutical Co., Ltd., written in ese, and is prepared for the information disclosure
Earnings of 3Q FY2011/3
 Earnings of 3Q FY2011/3 (April 1 December 31, 2010) January 28, 2011 FORWARDLOOKING STATEMENTS Forwardlooking statements such as those relating to earnings forecasts and other projections contained in
Earnings of 3Q FY2011/3 (April 1 December 31, 2010) January 28, 2011 FORWARDLOOKING STATEMENTS Forwardlooking statements such as those relating to earnings forecasts and other projections contained in
FYE March 2019 First Quarter Financial Highlights
 FYE March 2019 First Quarter Financial Highlights NAGASE & CO., LTD. August 3, 2018 Agenda Consolidated Statements of Income 3 Net Sales by Region (Domestic, Overseas) 4 Net Sales: Two Year Comparison
FYE March 2019 First Quarter Financial Highlights NAGASE & CO., LTD. August 3, 2018 Agenda Consolidated Statements of Income 3 Net Sales by Region (Domestic, Overseas) 4 Net Sales: Two Year Comparison
Investor Meeting on Q1 FY2017 Results
 Investor Meeting on FY2017 Results Kazuo Koshiji Senior Corporate Officer Chief Financial Officer (CFO) Head of Finance Division August 1, 2017 Copyright 2017 Santen Pharmaceutical Co., Ltd. All rights
Investor Meeting on FY2017 Results Kazuo Koshiji Senior Corporate Officer Chief Financial Officer (CFO) Head of Finance Division August 1, 2017 Copyright 2017 Santen Pharmaceutical Co., Ltd. All rights
Consolidated Financial Report for FY2000 Half-Year (April 1, 2000 September 30, 2000)
 Consolidated Financial Report for FY2000 Half-Year (April 1, 2000 September 30, 2000) October 26, 2000 Fuji Electric Co., Ltd. Summary of Consolidated Financial Results 1. Summary of consolidated statements
Consolidated Financial Report for FY2000 Half-Year (April 1, 2000 September 30, 2000) October 26, 2000 Fuji Electric Co., Ltd. Summary of Consolidated Financial Results 1. Summary of consolidated statements
INSTITUTIONAL RESEARCH Specialty Pharma COMPANY UPDATE Member FINRA/SIPC
 INSTITUTIONAL RESEARCH Specialty Pharma COMPANY UPDATE Member FINRA/SIPC Toll Free: 561-391-5555 www.dawsonjames.com 1 North Federal Highway - Suite 500 Boca Raton, FL 33432 Pulmatrix (Nasdaq/PULM) BUY
INSTITUTIONAL RESEARCH Specialty Pharma COMPANY UPDATE Member FINRA/SIPC Toll Free: 561-391-5555 www.dawsonjames.com 1 North Federal Highway - Suite 500 Boca Raton, FL 33432 Pulmatrix (Nasdaq/PULM) BUY
Double-Digit Non-GAAP EPS Growth in Second Quarter 2011: Non-GAAP EPS of $0.95; GAAP EPS of $0.65
 1 sur 8 29/07/2011 12:56 Merck Announces Second Quarter 2011 Financial Results Double-Digit Non-GAAP EPS Growth in Second Quarter 2011: Non-GAAP EPS of $0.95; GAAP EPS of $0.65 Total Company and Pharmaceutical
1 sur 8 29/07/2011 12:56 Merck Announces Second Quarter 2011 Financial Results Double-Digit Non-GAAP EPS Growth in Second Quarter 2011: Non-GAAP EPS of $0.95; GAAP EPS of $0.65 Total Company and Pharmaceutical
Third quarter dividends per share (yen) Second quarter dividends per share (yen)
 [ Disclaimer : The following is meant to be an accurate translation from the original Financial Report of Santen Pharmaceutical Co., Ltd., written in Japanese, and is prepared for the information disclosure
[ Disclaimer : The following is meant to be an accurate translation from the original Financial Report of Santen Pharmaceutical Co., Ltd., written in Japanese, and is prepared for the information disclosure
Strategic Roadmap Update and FY2015 Q1 Consolidated Financial Results Important notice Forward-Looking Statements Medical Information
 Strategic Roadmap Update and FY2015 Q1 Consolidated Financial Results Christophe Weber, President & CEO Rudolf van Houten, Acting CFO & Group Financial Controller July 30, 2015 Important notice Forward-Looking
Strategic Roadmap Update and FY2015 Q1 Consolidated Financial Results Christophe Weber, President & CEO Rudolf van Houten, Acting CFO & Group Financial Controller July 30, 2015 Important notice Forward-Looking
Operations and Financial Position
 Operations and Financial Position n Adoption of International Financial Reporting Standards (IFRS) Daiichi Sankyo and its consolidated subsidiaries ( the Group ) have adopted IFRS starting in the fiscal
Operations and Financial Position n Adoption of International Financial Reporting Standards (IFRS) Daiichi Sankyo and its consolidated subsidiaries ( the Group ) have adopted IFRS starting in the fiscal
Q results. 27 July 2016
 Q2 2016 results 27 July 2016 Cautionary statement regarding forward-looking statements This presentation may contain forward-looking statements. Forward-looking statements give the Group s current expectations
Q2 2016 results 27 July 2016 Cautionary statement regarding forward-looking statements This presentation may contain forward-looking statements. Forward-looking statements give the Group s current expectations
Financial Review. Financial Information and Data. Overview of the Year Ended March 31, 2017 (Fiscal 2016) Sales. Sales by Region
 Financial Review Overview of the Year Ended March 31, 2017 (Fiscal 2016) In its consolidated operating results (core basis) for fiscal 2016 Astellas posted a decrease in sales and increases in core operating
Financial Review Overview of the Year Ended March 31, 2017 (Fiscal 2016) In its consolidated operating results (core basis) for fiscal 2016 Astellas posted a decrease in sales and increases in core operating
Summary of Financial Statements for the Three Month Period Ended June 30, 2017 (IFRS, Consolidated) July 28, 2017
 Period Ended (IFRS, Consolidated) July 28, 2017 Takeda Pharmaceutical Company Limited Stock exchange listings: Tokyo, Nagoya, Fukuoka, Sapporo TSE Code: 4502 URL: http://www.takeda.co.jp Representative:
Period Ended (IFRS, Consolidated) July 28, 2017 Takeda Pharmaceutical Company Limited Stock exchange listings: Tokyo, Nagoya, Fukuoka, Sapporo TSE Code: 4502 URL: http://www.takeda.co.jp Representative:
CMIC HOLDINGS Co., Ltd. Consolidated Financial Results
 (Note) This translation is prepared and provided for readers' convenience only. In the event of any discrepancy between this translated document and the original Japanese document, the original document
(Note) This translation is prepared and provided for readers' convenience only. In the event of any discrepancy between this translated document and the original Japanese document, the original document
Settlement for the year ended March 31, 2006
 Settlement for the year ended March 31, 2006 May 15, 2006 -1- Contents 1 Business forecast 2 Financial results of significant consolidated subsidiaries 3 Consolidated
Settlement for the year ended March 31, 2006 May 15, 2006 -1- Contents 1 Business forecast 2 Financial results of significant consolidated subsidiaries 3 Consolidated
Financial Results of Astellas for the First Nine Months of FY2017
 Contact: Corporate Communications, Astellas Pharma Inc. TEL +81-3-3244-3201 January 31, 2018 Financial Results of Astellas for the First Nine Months of FY2017 Japan, January 31, 2018 Astellas Pharma Inc.
Contact: Corporate Communications, Astellas Pharma Inc. TEL +81-3-3244-3201 January 31, 2018 Financial Results of Astellas for the First Nine Months of FY2017 Japan, January 31, 2018 Astellas Pharma Inc.
3rd Quarter of FY2015 Business Results
 3rd Quarter of FY2015 Business Results (April December, 2015) February 3, 2016 Eizo Tabaru Board Director, Managing Executive officer General Manager of Finance & Accounting Dept. Overview of Q3 FY2015
3rd Quarter of FY2015 Business Results (April December, 2015) February 3, 2016 Eizo Tabaru Board Director, Managing Executive officer General Manager of Finance & Accounting Dept. Overview of Q3 FY2015
(Figures are rounded down to the nearest million yen) 1. Consolidated Performance for the nine months ended December 31, 2013
 [ Disclaimer: The following is meant to be an accurate translation from the original Financial Report of Santen Pharmaceutical Co., Ltd., written in Japanese, and is prepared for the information disclosure
[ Disclaimer: The following is meant to be an accurate translation from the original Financial Report of Santen Pharmaceutical Co., Ltd., written in Japanese, and is prepared for the information disclosure
FY4/14 Results Briefing
 Results Briefing June 5,2014 Result Overview 1 Consolidated P/L The Group reported net sales of 170,225 million, an increase of 10.1% year on year, reflecting the opening of new dispensing pharmacies and
Results Briefing June 5,2014 Result Overview 1 Consolidated P/L The Group reported net sales of 170,225 million, an increase of 10.1% year on year, reflecting the opening of new dispensing pharmacies and
FYE March 2017 Second Quarter Financial Briefing
 FYE March 2017 Second Quarter Financial Briefing November 25, 2016 Conte nts Contents FYE March 2017 Second Quarter Results P.3 FYE March 2017 Earnings Projections P.13 Progress of Mid-Term Management
FYE March 2017 Second Quarter Financial Briefing November 25, 2016 Conte nts Contents FYE March 2017 Second Quarter Results P.3 FYE March 2017 Earnings Projections P.13 Progress of Mid-Term Management
Financial Results for Fiscal Year 2017 (Consolidated) May 9, 2018 Name of Listed Company: SHIONOGI & CO., LTD.
 r Fiscal Year 2017 (Consolidated) May 9, 2018 Name of Listed Company: TD. Listed Exchanges: Section I of Tokyo Code: 4507 URL: http://www.shionogi.co.jp Representative: Isao Teshirogi, President and CEO
r Fiscal Year 2017 (Consolidated) May 9, 2018 Name of Listed Company: TD. Listed Exchanges: Section I of Tokyo Code: 4507 URL: http://www.shionogi.co.jp Representative: Isao Teshirogi, President and CEO
Bando Chemical Industries, Ltd.
 Consolidated Business Results for the First Half of Fiscal 2011 November 4, 2011 Bando Chemical Industries, Ltd. 0 First Half Financial Summary for the Fiscal Year Ending March 31, 2012 1 (1)Settlement
Consolidated Business Results for the First Half of Fiscal 2011 November 4, 2011 Bando Chemical Industries, Ltd. 0 First Half Financial Summary for the Fiscal Year Ending March 31, 2012 1 (1)Settlement
Financial Results - For the FYE March May 16, 2017
 Financial Results - For the FYE March 2017 - May 16, 2017 Contents 1. Overview for the FYE March 2017 2. Outlook for the FYE March 2018 3. The Meiji Group 2026 Vision (outline) Business forecasts and other
Financial Results - For the FYE March 2017 - May 16, 2017 Contents 1. Overview for the FYE March 2017 2. Outlook for the FYE March 2018 3. The Meiji Group 2026 Vision (outline) Business forecasts and other
Consolidated Financial Highlights
 Disclaimer Regarding Forward-looking Statements Any statements in this document, other than those of historical fact, are forward-looking statements about the future performance of EIZO and its group companies,
Disclaimer Regarding Forward-looking Statements Any statements in this document, other than those of historical fact, are forward-looking statements about the future performance of EIZO and its group companies,
1. Qualitative information on consolidated financial results for the first three months of (1) Information on business performance Consolidated busine
 Contact: Corporate Communications, Astellas Pharma Inc. TEL +81-3-3244-3201 August 1, 2013 Financial Results of Astellas for the First Three Months of Japan, August 1, 2013 Astellas Pharma Inc. (hereinafter
Contact: Corporate Communications, Astellas Pharma Inc. TEL +81-3-3244-3201 August 1, 2013 Financial Results of Astellas for the First Three Months of Japan, August 1, 2013 Astellas Pharma Inc. (hereinafter
Consolidated Financial Results FY2015 Q2
 Consolidated Financial Results FY2015 Q2 October 30, 2015 Rudolf van Houten Acting CFO & Group Financial Controller Important Notice Forward-Looking Statements This presentation contains forward-looking
Consolidated Financial Results FY2015 Q2 October 30, 2015 Rudolf van Houten Acting CFO & Group Financial Controller Important Notice Forward-Looking Statements This presentation contains forward-looking
CMIC HOLDINGS Co., Ltd. Consolidated Financial Results
 (Note) This translation is prepared and provided for readers' convenience only. In the event of any discrepancy between this translated document and the original Japanese document, the original document
(Note) This translation is prepared and provided for readers' convenience only. In the event of any discrepancy between this translated document and the original Japanese document, the original document
Consolidated Financial Highlights. Fiscal Year Ended Mar 31, 2006
 Consolidated Financial Highlights Fiscal Year Ended Mar 31, 2006 Table of Contents 1. Consolidated Statements of Income 2. Consolidated Balance Sheets 3. Selling, general and administrative expenses 4.
Consolidated Financial Highlights Fiscal Year Ended Mar 31, 2006 Table of Contents 1. Consolidated Statements of Income 2. Consolidated Balance Sheets 3. Selling, general and administrative expenses 4.
Fiscal 2009 Financial Results. May 10, 2010
 Fiscal 2009 Financial May 10, 2010 Forward-Looking Statements This presentation contains forward-looking statements. These statements are based on expectations in light of the information currently available,
Fiscal 2009 Financial May 10, 2010 Forward-Looking Statements This presentation contains forward-looking statements. These statements are based on expectations in light of the information currently available,
InDex Pharmaceuticals Holding AB (publ)
 InDex Pharmaceuticals Holding AB (publ) Interim report January-March 2018 Novel formulation for oral administration of cobitolimod PERIOD JANUARY-MARCH 2018 Revenues amounted to SEK 0.1 (0.0) million Operating
InDex Pharmaceuticals Holding AB (publ) Interim report January-March 2018 Novel formulation for oral administration of cobitolimod PERIOD JANUARY-MARCH 2018 Revenues amounted to SEK 0.1 (0.0) million Operating
Consolidated Financial Statements for the First Three Months of the March 31, 2011 Fiscal Year <under Japanese GAAP> July 30, 2010
 Consolidated Financial Statements for the First Three Months of the March 31, 2011 Fiscal Year July 30, 2010 Listed Company Name: TAISHO PHARMACEUTICAL CO., LTD. Stock Listing: TSE
Consolidated Financial Statements for the First Three Months of the March 31, 2011 Fiscal Year July 30, 2010 Listed Company Name: TAISHO PHARMACEUTICAL CO., LTD. Stock Listing: TSE
Management s Discussion and Analysis of Financial Condition and Results of Operations
 of Financial Condition and Results of Operations (Dollar amounts in thousands) For the fiscal year ended March 31, 2006, despite the loss of $635,960 of Celexa sales as compared with fiscal 2005, total
of Financial Condition and Results of Operations (Dollar amounts in thousands) For the fiscal year ended March 31, 2006, despite the loss of $635,960 of Celexa sales as compared with fiscal 2005, total
Investor Meeting on FY2015 Results and FY2016 Forecast
 Investor Meeting on FY2015 Results and FY2016 Forecast Akira Kurokawa President & CEO May 12, 2016 Copyright 2016 Santen Pharmaceutical Co., Ltd. All rights reserved. Santen s Corporate Values By focusing
Investor Meeting on FY2015 Results and FY2016 Forecast Akira Kurokawa President & CEO May 12, 2016 Copyright 2016 Santen Pharmaceutical Co., Ltd. All rights reserved. Santen s Corporate Values By focusing
Consolidated Financial Results for the 1 st Half of FYE 2019
 Consolidated Financial Results for the 1 st Half of SUBARU CORPORATION Toshiaki Okada Corporate Executive Vice President & CFO November 5 th, 2018 1 Summary Consolidated Financial Results for the 1 st
Consolidated Financial Results for the 1 st Half of SUBARU CORPORATION Toshiaki Okada Corporate Executive Vice President & CFO November 5 th, 2018 1 Summary Consolidated Financial Results for the 1 st
Conference Call on Q3 FY2018 Results
 Conference Call on FY2018 Results Shigeo Taniuchi President and Chief Operating Officer February 5, 2019 Copyright 2019 Santen Pharmaceutical Co., Ltd. All rights reserved. 2 Santen s Values and Mission
Conference Call on FY2018 Results Shigeo Taniuchi President and Chief Operating Officer February 5, 2019 Copyright 2019 Santen Pharmaceutical Co., Ltd. All rights reserved. 2 Santen s Values and Mission
Consolidated Financial Results for the 1st Quarter of Fiscal Year 2014
 Consolidated Financial Results for the 1st Quarter of Fiscal Year 2014 François-Xavier Roger Chief Financial Officer August 1, 2014 Notes on Disclosure Shift to IFRS and Core earnings From Q1 2014 Takeda
Consolidated Financial Results for the 1st Quarter of Fiscal Year 2014 François-Xavier Roger Chief Financial Officer August 1, 2014 Notes on Disclosure Shift to IFRS and Core earnings From Q1 2014 Takeda
To Shareholders. Interim Report for the First Six Months of Fiscal Year 2016
 To Our Shareholders I thank you sincerely for your ongoing support. Here, we provide an overview of the Company s operating performance during the first six months of fiscal year 216. To Shareholders Interim
To Our Shareholders I thank you sincerely for your ongoing support. Here, we provide an overview of the Company s operating performance during the first six months of fiscal year 216. To Shareholders Interim
Supplementary Data for FY2013 Business Results (From
 Supplementary Data for Business Results (From April 1,2012 to March 31, 2013 ) * "FY ended March 31" "JSR's accounting period (fiscal year) is defined as the period from April 1 to March 31 of the following
Supplementary Data for Business Results (From April 1,2012 to March 31, 2013 ) * "FY ended March 31" "JSR's accounting period (fiscal year) is defined as the period from April 1 to March 31 of the following
Consolidated Financial Results for FYE 2018
 Consolidated Financial Results for SUBARU CORPORATION Toshiaki Okada Corporate Executive Vice President & CFO May 11 th, 20180 0 Summary Consolidated Financial Results for Net sales and global unit sales
Consolidated Financial Results for SUBARU CORPORATION Toshiaki Okada Corporate Executive Vice President & CFO May 11 th, 20180 0 Summary Consolidated Financial Results for Net sales and global unit sales
Supplemental Documents for Fiscal Year May 15, 2015 Nippon Suisan Kaisha, Ltd.
 Supplemental Documents for Fiscal Year 214 May 15, 215 Nippon Suisan Kaisha, Ltd. Overview of FY214 Both revenue and income increased in Marine Products and Food Products. Revenue and income decreased
Supplemental Documents for Fiscal Year 214 May 15, 215 Nippon Suisan Kaisha, Ltd. Overview of FY214 Both revenue and income increased in Marine Products and Food Products. Revenue and income decreased
Half-Year Report 2007
 July 25, 2007 Who we are Half-Year highlights Cosmo Pharmaceuticals SpA is a pharmaceutical company headquartered in Lainate, Milan, Italy, that is listed on the SWX Swiss Stock Exchange (SWX:COPN). It
July 25, 2007 Who we are Half-Year highlights Cosmo Pharmaceuticals SpA is a pharmaceutical company headquartered in Lainate, Milan, Italy, that is listed on the SWX Swiss Stock Exchange (SWX:COPN). It
Consolidated Financial Results for the First Quarter of the Fiscal Year Ending September 30, 2018 (Three Months Ended December 31, 2017)
 Consolidated Financial Results for the First Quarter of the Fiscal Year Ending September 30, 2018 (Three Months Ended December 31, 2017) Company name: Fuji Pharma Co., Ltd. Stock code: 4554 (URL: http://www.fujipharma.jp)
Consolidated Financial Results for the First Quarter of the Fiscal Year Ending September 30, 2018 (Three Months Ended December 31, 2017) Company name: Fuji Pharma Co., Ltd. Stock code: 4554 (URL: http://www.fujipharma.jp)
Forward-Looking Statements. Consolidated Financial Results for the 3rd Quarter of Fiscal Year François-Xavier Roger Chief Financial Officer
 Consolidated Financial Results for the 3rd Quarter of Fiscal Year 2014 François-Xavier Roger Chief Financial Officer February 5, 2015 Forward-Looking Statements This presentation contains forward-looking
Consolidated Financial Results for the 3rd Quarter of Fiscal Year 2014 François-Xavier Roger Chief Financial Officer February 5, 2015 Forward-Looking Statements This presentation contains forward-looking
Reference Data (Consolidated Financial Results for 1QFY2007)
 Stock code number 4568 Reference Data (Consolidated Financial Results for ) [1]Summary of Financial Statement P1 [2]Currency Rate P1 [3]Consolidated sales of Global Product P2 [4]Overseas Sales P2 [5]Consolidated
Stock code number 4568 Reference Data (Consolidated Financial Results for ) [1]Summary of Financial Statement P1 [2]Currency Rate P1 [3]Consolidated sales of Global Product P2 [4]Overseas Sales P2 [5]Consolidated
Investor Meeting on Q2 FY2016 Results
 Investor Meeting on Q2 FY2016 Results Akira Kurokawa President & CEO November 2016 Copyright 2016 Santen Pharmaceutical Co., Ltd. All rights reserved. Santen s Corporate Values By focusing on ophthalmology,
Investor Meeting on Q2 FY2016 Results Akira Kurokawa President & CEO November 2016 Copyright 2016 Santen Pharmaceutical Co., Ltd. All rights reserved. Santen s Corporate Values By focusing on ophthalmology,
ACTELION LTD FIRST QUARTER 2015 FINANCIAL REPORT.
 ACTELION LTD FIRST QUARTER 2015 FINANCIAL REPORT. APRIL 21, 2015 2 CONTENTS 03 FIRST QUARTER 2015 FINANCIAL REVIEW 15 UNAUDITED FIRST QUARTER 2015 CONSOLIDATED FINANCIAL STATEMENTS Disclaimer and notes
ACTELION LTD FIRST QUARTER 2015 FINANCIAL REPORT. APRIL 21, 2015 2 CONTENTS 03 FIRST QUARTER 2015 FINANCIAL REVIEW 15 UNAUDITED FIRST QUARTER 2015 CONSOLIDATED FINANCIAL STATEMENTS Disclaimer and notes
JUBILANT LIFE SCIENCES Q2 & H1 FY19 RESULTS
 PRESS RELEASE Noida, Monday, Oct 22, 2018 Jubilant Life Sciences Ltd. 1A, Sector 16A, Noida 201301, India Tel.: +91 120 4361000 http://www.jubl.com JUBILANT LIFE SCIENCES Q2 & H1 FY19 RESULTS JUBILANT
PRESS RELEASE Noida, Monday, Oct 22, 2018 Jubilant Life Sciences Ltd. 1A, Sector 16A, Noida 201301, India Tel.: +91 120 4361000 http://www.jubl.com JUBILANT LIFE SCIENCES Q2 & H1 FY19 RESULTS JUBILANT
Precision System Science Co., Ltd.
 Nov 14, 2017 Precision System Science Co., Ltd. SUMMARY OF CONSOLIDATED FINANCIAL STATEMENTS For the First Three Months of the Fiscal Year, Ending June 30 2018 (From July 1, 2017 to September 30, 2017)
Nov 14, 2017 Precision System Science Co., Ltd. SUMMARY OF CONSOLIDATED FINANCIAL STATEMENTS For the First Three Months of the Fiscal Year, Ending June 30 2018 (From July 1, 2017 to September 30, 2017)
Caution concerning Forward-Looking Statements Purpose of This Material and Cautionary Notes This material is prepared for the purpose of understanding
 (Reference Material) Voluntary Adoption of International Financial Reporting Standards(IFRS)from the fiscal year ended March 31,2012 Japan Tobacco Inc. Caution concerning Forward-Looking Statements Forward-Looking
(Reference Material) Voluntary Adoption of International Financial Reporting Standards(IFRS)from the fiscal year ended March 31,2012 Japan Tobacco Inc. Caution concerning Forward-Looking Statements Forward-Looking
Financial Results for the Fiscal Year Ended March 31, 2017 (FY2016) and Guidance for the Fiscal Year Ending March 31, 2018 (FY2017)
 Financial Results for the Fiscal Year Ended March 31, 2017 (FY2016) and Guidance for the Fiscal Year Ending March 31, 2018 (FY2017) Terumo Corporation Managing Executive Officer, Investor Relations, Corporate
Financial Results for the Fiscal Year Ended March 31, 2017 (FY2016) and Guidance for the Fiscal Year Ending March 31, 2018 (FY2017) Terumo Corporation Managing Executive Officer, Investor Relations, Corporate
Photocure ASA - Third Quarter Report 2008
 Photocure ASA - Third Quarter Report 2008 Highlights Sales revenues increase 52% to NOK 23.6 million ( from NOK 15.6 million in third quarter 2007) Operating profit improved by NOK 13.7 million to NOK
Photocure ASA - Third Quarter Report 2008 Highlights Sales revenues increase 52% to NOK 23.6 million ( from NOK 15.6 million in third quarter 2007) Operating profit improved by NOK 13.7 million to NOK
Orion Interim Report Q1 Q2/2012
 Orion Interim Report Q1 Q2/2012 31 July 2012 Timo Lappalainen President and CEO This presentation contains forward-looking statements which involve risks and uncertainty factors. These statements are not
Orion Interim Report Q1 Q2/2012 31 July 2012 Timo Lappalainen President and CEO This presentation contains forward-looking statements which involve risks and uncertainty factors. These statements are not
Performance Summary. Copyright 2018 Harmonic Drive Systems Inc.
 Performance Summary 2 1st-half consolidated results (versus original forecasts for the period) Original forecasts (announced May 11) 1st-half FY ending Mar.31,2019 Vs. original forecasts for the period
Performance Summary 2 1st-half consolidated results (versus original forecasts for the period) Original forecasts (announced May 11) 1st-half FY ending Mar.31,2019 Vs. original forecasts for the period
Eurand Reports Recent Developments and Fourth Quarter and Full-Year 2008 Financial Results
 Eurand Reports Recent Developments and Fourth Quarter and Full-Year 2008 Financial Results AMSTERDAM, THE NETHERLANDS, Mar 05, 2009 (MARKET WIRE via COMTEX News Network) -- Eurand N.V. (NASDAQ: EURX) Recent
Eurand Reports Recent Developments and Fourth Quarter and Full-Year 2008 Financial Results AMSTERDAM, THE NETHERLANDS, Mar 05, 2009 (MARKET WIRE via COMTEX News Network) -- Eurand N.V. (NASDAQ: EURX) Recent
FY 2008 First Quarter Results. August 4, 2008 Santen Pharmaceutical Co., Ltd
 FY 2008 First Quarter Results August 4, 2008 Santen Pharmaceutical Co., Ltd 1 FY 2008 First Quarter Consolidated Financial Results Chief Financial Officer Yoshihiro Noutsuka Forward-looking statements:
FY 2008 First Quarter Results August 4, 2008 Santen Pharmaceutical Co., Ltd 1 FY 2008 First Quarter Consolidated Financial Results Chief Financial Officer Yoshihiro Noutsuka Forward-looking statements:
Investor Meeting on FY2015 Third Quarter Results and Revised FY2015 Forecasts Kazuo Koshiji
 Investor Meeting on FY2015 Third Quarter Results and Revised FY2015 Forecasts Kazuo Koshiji Senior Corporate Officer Chief Financial Officer Head of Finance Division February 2, 2016 Santen s Corporate
Investor Meeting on FY2015 Third Quarter Results and Revised FY2015 Forecasts Kazuo Koshiji Senior Corporate Officer Chief Financial Officer Head of Finance Division February 2, 2016 Santen s Corporate
Financial Results Analysis Quarter & 9 Months Ended December 31, 2011
 Financial Results Analysis Quarter & 9 Months Ended December 31, 2011 Disclaimer 1 Statements in this document relating to future status, events, or circumstances, including but not limited to statements
Financial Results Analysis Quarter & 9 Months Ended December 31, 2011 Disclaimer 1 Statements in this document relating to future status, events, or circumstances, including but not limited to statements
Gulliver International Co., Ltd.
 Gulliver International Co., Ltd. Non-consolidated Results Third Quarter of the Fiscal year Ending February 28, 2006 (Nine month period ended November 30, ) This document has been translated from the original
Gulliver International Co., Ltd. Non-consolidated Results Third Quarter of the Fiscal year Ending February 28, 2006 (Nine month period ended November 30, ) This document has been translated from the original
Gulliver International Co., Ltd.
 Gulliver International Co., Ltd. Non-Consolidated Results First Quarter of the Fiscal Year Ending February 29, 2008 (Three-month period ended May 31, ) This document has been translated from the original
Gulliver International Co., Ltd. Non-Consolidated Results First Quarter of the Fiscal Year Ending February 29, 2008 (Three-month period ended May 31, ) This document has been translated from the original
Precision System Science Co., Ltd.
 Aug 14, 2017 Precision System Science Co., Ltd. SUMMARY OF CONSOLIDATED FINANCIAL STATEMENTS Fiscal Year, Ended June 30 2017 (From July 1, 2016 to June 30, 2017) The English Edition is digested translation
Aug 14, 2017 Precision System Science Co., Ltd. SUMMARY OF CONSOLIDATED FINANCIAL STATEMENTS Fiscal Year, Ended June 30 2017 (From July 1, 2016 to June 30, 2017) The English Edition is digested translation
FY4/13 Results Briefing
 Results Briefing June 5,2013 Results Overview 1 Consolidated P/L The Group reported net sales of 154,560 million, an increase of 8.2% year on year due to the opening of 83 stores including M&A. However,
Results Briefing June 5,2013 Results Overview 1 Consolidated P/L The Group reported net sales of 154,560 million, an increase of 8.2% year on year due to the opening of 83 stores including M&A. However,
Semiannual Consolidated Results for the Fiscal Year Ending March 31, 2005
 4503 Yamanouchi Pharmaceutical Co., Ltd. Semiannual Consolidated Results for the Fiscal Year Ending March 31, 2005 1. Change in Accounting Policies 1. Consolidation Number of Consolidated Subsidiaries:
4503 Yamanouchi Pharmaceutical Co., Ltd. Semiannual Consolidated Results for the Fiscal Year Ending March 31, 2005 1. Change in Accounting Policies 1. Consolidation Number of Consolidated Subsidiaries:
Business Results for The First Quarter of FY2018 (Three-month period ended June 30, 2018) July 31, 2018
 Business Results for The First Quarter of FY218 (Three-month period ended June 3, 218) July 31, 218 Please be aware of the following: * The financial information provided on this material has been prepared
Business Results for The First Quarter of FY218 (Three-month period ended June 3, 218) July 31, 218 Please be aware of the following: * The financial information provided on this material has been prepared
Second Quarter of FY2008 (March 2009) Earnings Results. Akira Uehara, President Taisho Pharmaceutical October 30, 2008
 Second Quarter of FY2008 (March 2009) Earnings Results Akira Uehara, President Taisho Pharmaceutical October 30, 2008 Results for the 2Q of Fiscal 2008 2nd quarter results (cumulative) (Yen B) FY07 2Q
Second Quarter of FY2008 (March 2009) Earnings Results Akira Uehara, President Taisho Pharmaceutical October 30, 2008 Results for the 2Q of Fiscal 2008 2nd quarter results (cumulative) (Yen B) FY07 2Q
Company presentation. 2 nd Annual Specialty Pharmaceuticals Conference. Salomon Smith Barney New York, 7 March 2002
 Company presentation 2 nd Annual Specialty Pharmaceuticals Conference Salomon Smith Barney New York, 7 March 2002 Recordati an excellent marketing company with productive original research Growth drivers
Company presentation 2 nd Annual Specialty Pharmaceuticals Conference Salomon Smith Barney New York, 7 March 2002 Recordati an excellent marketing company with productive original research Growth drivers
Medium-Term Business Plan (Revised Version) Year ended March 31, 2016 ~ Year ending March 31, 2018
 Medium-Term Business Plan (Revised Version) March 31, 2016 ~ August, 2016 Medium-term Business Plan -M1 Trust 2018 - Background to Revision of the Plan 1) Changes in market conditions surrounding the generics
Medium-Term Business Plan (Revised Version) March 31, 2016 ~ August, 2016 Medium-term Business Plan -M1 Trust 2018 - Background to Revision of the Plan 1) Changes in market conditions surrounding the generics
KORRES GROUP FY 2016 FINANCIAL RESULTS
 KORRES GROUP FY 2016 FINANCIAL RESULTS FY 2016 Major Highlights Marginal growth in Total Consolidated Sales +4% growth in Sales in Greece +18% Growth in Key international markets: Germany, Norway and UK
KORRES GROUP FY 2016 FINANCIAL RESULTS FY 2016 Major Highlights Marginal growth in Total Consolidated Sales +4% growth in Sales in Greece +18% Growth in Key international markets: Germany, Norway and UK
CMIC HOLDINGS Co., Ltd. Consolidated Financial Results
 (Note) This translation is prepared and provided for readers' convenience only. In the event of any discrepancy between this translated document and the original Japanese document, the original document
(Note) This translation is prepared and provided for readers' convenience only. In the event of any discrepancy between this translated document and the original Japanese document, the original document
Kyowa Hakko Kirin Co, Ltd
 Kyowa Hakko Kirin Co, Ltd Consolidated Financial Summary First Quarter of the Fiscal Period Ending December 2009 (April 1, 2009 June 30, 2009) This document is an English translation of parts of the Japanese-language
Kyowa Hakko Kirin Co, Ltd Consolidated Financial Summary First Quarter of the Fiscal Period Ending December 2009 (April 1, 2009 June 30, 2009) This document is an English translation of parts of the Japanese-language
Vectura Group plc Interim Report and Accounts. for the six months ended 30 September 2012
 Vectura Group plc Interim Report and Accounts for the six months ended 30 September 2012 02 Vectura Group plc Interim Report and Accounts 2012 2 Contents 01 Highlights 02 Chief Executive s statement 03
Vectura Group plc Interim Report and Accounts for the six months ended 30 September 2012 02 Vectura Group plc Interim Report and Accounts 2012 2 Contents 01 Highlights 02 Chief Executive s statement 03
Apr-Jun Apr-Jun. (billion yen) (billion yen) (billion yen) % (billion yen)
 First Quarter of Fiscal 2012 Consolidated Financial Results Hiroshi Takahara Senior Vice President Corporate Finance & Controlling Department July 30, 2012 Consolidated Financial Results for the First
First Quarter of Fiscal 2012 Consolidated Financial Results Hiroshi Takahara Senior Vice President Corporate Finance & Controlling Department July 30, 2012 Consolidated Financial Results for the First
Summary of Consolidated Financial Results for the Third Quarter of the Year Ending March 31, 2018[Japanese GAAP](Unaudited)
 Summary of Consolidated Financial Results for the Third Quarter of the Year Ending March 31, 2018[Japanese GAAP](Unaudited)](/thumbs/81/82606956.jpg) Summary of Consolidated Financial Results for the Third Quarter of the Year Ending March 31, 2018[Japanese GAAP](Unaudited) January 30, 2018 Company Name: SUMITOMO DAINIPPON PHARMA CO., LTD. Stock Exchange
Summary of Consolidated Financial Results for the Third Quarter of the Year Ending March 31, 2018[Japanese GAAP](Unaudited) January 30, 2018 Company Name: SUMITOMO DAINIPPON PHARMA CO., LTD. Stock Exchange
Consolidated Business Results For the Fiscal Year Ending March 31, 2012 Bando Chemical Industries, Ltd.
 Consolidated Business Results For the Fiscal Year Ending March 31, 2012 Bando Chemical Industries, Ltd. Financial Summary Fiscal Year Ending March 31, 2012 (1)Settlement of Accounts The production rates
Consolidated Business Results For the Fiscal Year Ending March 31, 2012 Bando Chemical Industries, Ltd. Financial Summary Fiscal Year Ending March 31, 2012 (1)Settlement of Accounts The production rates
Full Year Results th February 2012
 Full Year Results 2011 7 th February 2012 Sir Andrew Witty Chief Executive Officer Simon Dingemans Chief Financial Officer Delivering our strategy Grow a diversified global business Deliver more products
Full Year Results 2011 7 th February 2012 Sir Andrew Witty Chief Executive Officer Simon Dingemans Chief Financial Officer Delivering our strategy Grow a diversified global business Deliver more products
IR PRESENTATION June 2015
 IR PRESENTATION June 2015 Results Overview 1 Consolidated P/L For the fiscal year under the review, the Group reported net sales of 187,904 million, an increase of 10.4% year on year, reflecting the opening
IR PRESENTATION June 2015 Results Overview 1 Consolidated P/L For the fiscal year under the review, the Group reported net sales of 187,904 million, an increase of 10.4% year on year, reflecting the opening
Financial Results for the Fiscal Year ended March 31, 2018 (IFRS, Consolidated)
 Financial Results for the Fiscal Year ended March 31, 2018 (IFRS, Consolidated) Company name: Mitsubishi Tanabe Pharma Corporation Stock exchange listings: Tokyo Securities code number: 4508 URL: https://www.mt-pharma.co.jp/
Financial Results for the Fiscal Year ended March 31, 2018 (IFRS, Consolidated) Company name: Mitsubishi Tanabe Pharma Corporation Stock exchange listings: Tokyo Securities code number: 4508 URL: https://www.mt-pharma.co.jp/
Consolidated Financial Results for the 3rd Quarter of the Fiscal Year Ending March 31, 2010
 February 5, 2010 Consolidated Financial Results for the 3rd Quarter of the Fiscal Year Ending March 31, 2010 NIHON KOHDEN CORPORATION (6849) Stock Exchange Listing: Head Office: Representative: Contact:
February 5, 2010 Consolidated Financial Results for the 3rd Quarter of the Fiscal Year Ending March 31, 2010 NIHON KOHDEN CORPORATION (6849) Stock Exchange Listing: Head Office: Representative: Contact:
Briefing on Financial Statements for the First Half of the Year Ending March 2016
 Briefing on Financial Statements for the First Half of the Year Ending March 216 November 12, 215 http://www.tsugami.co.jp Copyright 215 TSUGAMI CORPORATION All rights reserved. 1. Business performance
Briefing on Financial Statements for the First Half of the Year Ending March 216 November 12, 215 http://www.tsugami.co.jp Copyright 215 TSUGAMI CORPORATION All rights reserved. 1. Business performance
Company presentation. Versailles, September 5 th, 2002
 Company presentation Versailles, September 5 th, 2002 Recordati an excellent marketing company with productive original research Growth drivers focused sales effort successful roll-out out of lercanidipine
Company presentation Versailles, September 5 th, 2002 Recordati an excellent marketing company with productive original research Growth drivers focused sales effort successful roll-out out of lercanidipine
3Q FY2018 Financial Results. February 4, 2019
 3Q Financial February 4, 2019 1 Contents 1. Review of 3Q Financial and Forecast 2. By Segment 3. Shareholder Return 1. Review of 3Q Financial and Forecast 2 3Q Financial and Forecast (Unit : Yen in billion)
3Q Financial February 4, 2019 1 Contents 1. Review of 3Q Financial and Forecast 2. By Segment 3. Shareholder Return 1. Review of 3Q Financial and Forecast 2 3Q Financial and Forecast (Unit : Yen in billion)
Forest Laboratories 2009 Annual Report
 Forest Laboratories 2009 Annual Report F OREST L ABORATORIES 2009 ANNUAL REPORT Forest Laboratories, Inc. Forest Laboratories develops, manufactures and markets pharmaceutical products principally in the
Forest Laboratories 2009 Annual Report F OREST L ABORATORIES 2009 ANNUAL REPORT Forest Laboratories, Inc. Forest Laboratories develops, manufactures and markets pharmaceutical products principally in the
Consolidated 11-year highlights
 2006.3 2007.3 2008.3 2009.3 1. Operating results Net sales 938,082 1,009,586 1,036,624 943,410 Gross profit 250,365 258,737 256,428 218,636 Percentage of net sales (%) 26.7 25.6 24.7 23.2 Operating income
2006.3 2007.3 2008.3 2009.3 1. Operating results Net sales 938,082 1,009,586 1,036,624 943,410 Gross profit 250,365 258,737 256,428 218,636 Percentage of net sales (%) 26.7 25.6 24.7 23.2 Operating income
Lupin Investor Presentation Q3FY14
 Lupin Investor Presentation Q3FY14 Vision: To be an innovation led transnational company Safe harbor statement Materials and information provided during this presentation may contain forward looking statements.
Lupin Investor Presentation Q3FY14 Vision: To be an innovation led transnational company Safe harbor statement Materials and information provided during this presentation may contain forward looking statements.
Pharmaxis Ltd ABN
 ABN 75 082 811 630 ASX Half year report 31 December 2009 Lodged with the ASX under Listing Rule 4.2A This report is to be read in conjunction with the financial statements for the year ended 30 June 2009
ABN 75 082 811 630 ASX Half year report 31 December 2009 Lodged with the ASX under Listing Rule 4.2A This report is to be read in conjunction with the financial statements for the year ended 30 June 2009
Investor Meeting on Q3 FY2017 Results
 Investor Meeting on FY2017 Results February 6, 2018 Copyright 2018 Santen Pharmaceutical Co., Ltd. All rights reserved. Today s Agenda 1. FY2017 Financial Results Kazuo Koshiji Senior Corporate Officer
Investor Meeting on FY2017 Results February 6, 2018 Copyright 2018 Santen Pharmaceutical Co., Ltd. All rights reserved. Today s Agenda 1. FY2017 Financial Results Kazuo Koshiji Senior Corporate Officer
