Q Second Quarter 2016
|
|
|
- Antony Roland Powers
- 5 years ago
- Views:
Transcription
1 Q Second Quarter 2016
2 Highlights for the second quarter of 2016 o Group sales increased with NOK 3.8 million from NOK 11.5 million in the first quarter of 2016 to NOK 15.3 million in the second quarter of 2016 o EBITDA was NOK -1.6 million in the second quarter of 2016 compared to NOK -1.0 million in the second quarter of 2015 as a result of increased spending in commercialization of Woulgan Gel o FDA filing of a 510K application for Woulgan for the US market o Woulgan commercialization process is gaining traction with a number of user trials, a new distributor in Germany and the first commercial sale in Finland Key financials Amount in NOK Q Q M M 2015 Sales EBITDA EBIT Net cash flow from operations Net cash end of period
3 Biotec Pharmacon Group Figures million (18.6) for the first six months of Biotec Pharmacon ASA, (hereinafter Biotec or, the Company ) reported sales of NOK 15.3 million (11.5) for the second quarter of EBITDA was NOK -1.6 million (-1.0) and EBIT NOK -2.1 million (-1.7) in the quarter. Net financial income was NOK 0 million (0.4), generating a loss before tax of NOK -2.1 million (-1.3) for the quarter. Reduction in EBITDA for the second quarter of 2016 compared to the same quarter last year is mainly due to increased commercial activities relating to the Woulgan Gel. The group had 40 employees at the end of the second quarter, compared to 36 employees at the end of the second quarter of Both the beta-glucan and the enzyme segment reported growth in sales compared to the second quarter of 2015, with sales of NOK 7.5 million and NOK 7.8 million respectively. For the first six months, sales increased to NOK 32.6 million, from 23.8 million in the first six months of 2015.The group had a gross contribution of NOK 9.7 million (9.0) in the second quarter of 2016 and a gross contribution of NOK 22.8 Balance Sheet Total equity amounted to NOK 81.6 million at the end of the second quarter of 2016 compared to NOK 86.7 million at the end of Total assets were NOK 91.1 million at the end of the second quarter of 2016, down from NOK million at the end of The Company has no interest-bearing debt. Cash Flow Net cash flow from operating activities was NOK -3.0 million in the second quarter of 2016, compared to NOK -4.8 million in the same quarter in The operating cash flow reflects a change in working capital of NOK 8.7 million compared to end of fourth quarter This is due to normal fluctuations in the working capital.
4 Shareholder matters The total number of issued shares was 43,944,673 at the end of the second quarter of The number of issued employee share options was 1,167,750 at the end of the quarter. In total 203,250 of these share options can be exercised in Net cash flow from investing and financing activities was NOK 0 in the second quarter and for the first six months of Changes in cash and cash equivalents were NOK -3.0 million in the second quarter and NOK million for the first six months. This generated a cash balance of NOK 64.7 million at the end of the quarter, compared to NOK 78.3 million at the end of Risk factors Biotec s business is exposed to a number of risk factors that may affect parts or all of the Company s activities. There are no substantial changes in the risk factors that are described in the annual report for 2015.
5 Business areas reporting: Beta-glucans Biotec Pharmacon continues to deliver on its short-term goals for Focus in the first six months has been on securing Key Opinion Leader (KOL) support and establishing market access in key regions for the Company s novel wound care product Woulgan. In addition, the Company seeks to continue to develop sales in the animal health and nutrition business. The Company is working closely with its distribution partner, H&R Healthcare, both in driving evaluations and promoting Woulgan at congresses. In addition, a UK launch plan is being prepared. The launch will be dependent on Woulgan receiving Drug Tariff approval. Woulgan - UK The clinical focus group successfully completed recruitment of additional 40 patients in the second quarter. The product has impressed clinicians by repeatedly demonstrating its effectiveness in reactivating stalled wounds across a variety of wound types, often in patients whose wounds have been stalled for 12 weeks or longer. The case series from the group will be submitted for publication in a UK wound journal later this year. The majority of clinicians from the focus group are enthusiastic and wish to continue working with Woulgan. This includes presenting on their experiences, continuing further evaluations, proposing care guidelines using Woulgan and adding Woulgan to their local prescribing guides (formularies) for stalled wounds. In total, over 70 patients have been treated with Woulgan in the UK during the first six months of Biotec continues to provide the NHS with information as they process Woulgan s Drug Tariff application. In a recent response to the NHS, Biotec were able to use data from the UK clinical focus group to demonstrate Woulgan s clinical effectiveness in different types of wound, and thereby supporting the positioning of the product as a therapy for stalled wounds in general. Woulgan - Nordic Our Nordic distributor Navamedic ASA sees interesting opportunities for Woulgan in the Nordic markets, based on clinician feedback from market evaluations. More than 170 Nordic patients have been treated with Woulgan in the first six months of Navamedic has already increased its Woulgan sales headcounts and more sales people are planned for the rest of Navamedic gained its first sales in Finland during the second quarter with more sales expected as more tenders are published after the summer. The Finnish team has offered Woulgan in tenders covering 70% of the market. As outlined in previous updates, when Woulgan is listed on a tender, it makes ordering the product in volume significantly more convenient and is thus a key requirement for larger market share in any region. In all regions, Navamedic is focused on adding Woulgan to tenders while it renews and builds clinician support to adopt Woulgan in parallel. Woulgan - Germany In June, the Company signed a distribution agreement with Rogg Verbanstoffe GmbH (hereinafter Rogg ) for Woulgan distribution to physician offices and pharmacies. Biotec will manage selected homecare companies and key
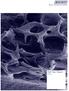
6 hospital activities directly. The agreement has an initial term of one year with the intention to prolong thereafter. Direct management of homecare companies and key hospitals offers potentially faster sales and earlier production of German-based evidence to both drive adoption and support reimbursement in the market. Biotec will support Rogg through its marketing division and has hired a commercial lead for Germany to coordinate commercial activities. have been presented to a selected group of wound care professionals for feedback on their appeal within the hospital and home care segments. Both product versions were perceived as novel, with a significant potential in wound care management. The Company continues to identify the optimal format for these two new product extensions. During the second quarter of 2016, four sites in the UK were recruited to the ongoing Post Market Clinical Follow-up study, in addition to the already procured Swedish site. The randomised controlled trial aims to recruit a total of 80 patients suffering from diabetic foot ulcers, of which 60 will be treated with Woulgan and 20 with the product Intrasite as the comparator. Picture 1: From EWMA, Bremen, May 2016 Woulgan - US In June 2016, Biotec submitted a 510K application for Woulgan to the FDA. This is the first step in a process to obtain a favourable commercial positioning in this important market. Such an application will normally take 6-9 months to process. In parallel, the Company is evaluating a partner process for the US. By involving a partner earlier in the commercialization, the Company will benefit from valuable insight and input to achieve a favourable product positioning for Woulgan in the US market. Woulgan - Other Two pilot versions of the new wound care products that are currently under development Beta-glucans - Other The ongoing clinical study at Memorial Sloan Kettering is recruiting patients at a high pace, 87 patients have been enrolled at end of second quarter of 2016 as compared to 65 at end of the first quarter. The neuroblastoma patients are treated with the combination of an experimental cancer vaccine developed by Memorial Sloan Kettering Cancer Centre and Soluble Beta Glucan (SBG ) from Biotec. SBG is used for its immunomodulatory properties. The study aims to recruit a total of 115 patients, whereof 100 are treated under phase II of the protocol focusing on not only safety but also efficacy. Biotec is exploring possibilities to provide products for other investigator-driven trials to gain more support for the application of SBG in cancer treatment. Biotec was cleared by the Court of Arbitration regarding the matter of the Company s nutrition business brought up by Sana Pharma AS. Sana Pharma has paid for costs incurred. Biotec continues the commercial relationship with Sana Pharma on a non-exclusive basis.
7 The Company has started to deliver its feed ingredient product M-Glucan to the new customer that signed a supply agreement in the first quarter of In general, there is an increasing demand for Biotec s proprietary betaglucan products used in the animal health and nutrition segment. In the second quarter of 2016, Biotec secured additional grants to conduct more studies to support the scientific documentation of its animal health product M-Glucan. Such documentation is important to grow the business and to support the uniqueness of the beta-glucans. Financial review beta-glucans BBG sales amounted to NOK 7.5 million in the second quarter of 2016, compared to NOK 4.2 million in the second quarter of Sales for the first six months was in total NOK 16.6 million, compared to NOK 8.9 million in the same period of The increase is mainly due to sales growth within the animal health and nutrition segment. Operating expenses increased from NOK 5.6 million in the second quarter of 2015 to NOK 7.7 million in the second quarter of 2016, mainly due to increases in personnel expenses and external services for the commercialization of Woulgan. Operating expenses for the first six months have increased from NOK 11.2 million in 2015 to NOK 17.5 million in Biotec expects this increase to continue as Woulgan is being launched and commercialized in several markets. EBITDA for the second quarter of 2016 was NOK -4.2 million compared to NOK -3.1 million in the same period last year.
8 Enzymes (ArcticZymes) Business ArcticZymes continues its first quarter momentum with a good second quarter, resulting in sales of NOK 7.8 million. Several new orders from existing customers, as well as new customers in pilot to scale-up phase contributed to the positive result in the second quarter. The first half 2016 result exceeded a positive first half In addition, a number of new customers submitted their first orders in Europe and America. This greatly reinforces the strategic objective to broaden the business through bringing on board new partners, which in hand mitigates risk and adds diversity to the Company s business. The Company has increased its focus on Europe, through the implementation of a dedicated European business development person at the beginning of ArcticZymes have been able to grow in scope and scale the number of existing key accounts in this territory. It is also prioritizing strategically relevant European prospects that are in the product development process. There is strong interest in our polymerase product developments from potential partners. To further support the polymerase initiatives, ArcticZymes has in collaboration with Norinnova Technology Transfer and University of Tromsø, been granted funding from the Research Council of Norway, through the FORNY program. The project MDxPol Marine DNA polymerases as tools for next generation Molecular Diagnostic solutions, aims to support phase II of our polymerase initiative, where ArcticZymes will bring several novel polymerases to the market during The strategy is to offer the customers a portfolio of slightly different polymerases, which will make it easier for them to select the most optimal enzyme for integration into their latest technologies and kit based products. R&D Polymerase Update An advanced prototype of our first polymerase enzyme is ready for customer testing. ArcticZymes plans to initiate testing with a selected handful of leading companies in Molecular Diagnostics and Next Generation Sequencing in the second half of Assuming prototype-testing goes well, the first commercial material will be made available to customers via our Early Access Program.
9 Financial review Enzymes Sales in ArcticZymes amounted to NOK 7.8 million in the second quarter of 2016, up from NOK 7.3 million in the same quarter last year. The Company s sales came from a limited number of larger orders. This will continue to give fluctuations in sales per quarter going forward. Operating expenses have increased from NOK 5.1 million in the second quarter of 2015 to NOK 5.9 million in the second quarter of 2016, mainly because of increased personnel expenses. Operating expenses for the first six months have moved from NOK 11.2 million in 2015 to NOK 12.7 million in Other revenues for the first six months of 2016 showed a marginal decrease from NOK 2.8 million in 2015 to NOK 2.6 million in 2016 Other revenues mainly derive from research grants, which decreased to NOK 1.4 million from NOK 1.5 million in the second quarter last year. EBITDA was a positive NOK 3.0 million in the second quarter of 2016, a slight reduction from NOK 3.3 million in the same quarter of 2015.
10 OUTLOOK Biotec is in a good position for creating shareholder value in the years to come, by dedicating resources in developing commercial value from the Company s key product platforms. Furthermore, the Company expects the enzyme market to grow and develop structurally over the next years, as the industry represents attractive opportunities for a wide array of partnerships. In wound care, focus is on positioning Woulgan as the key product for stalled wounds. This represents a market opportunity of at least USD 100 million in in-market sales for the Woulgan product platform. As earlier announced, the 2016 operational targets for Woulgan are: Entering into distribution agreement(s) for Woulgan in Germany Finalising the UK reimbursement process in the high-end category of the market Driving sales in the UK and the Nordic countries Continuing to develop an international sales support organization In addition, the Woulgan 510K submission for the US is now in process at the FDA. Such an application will normally take 6-9 months so the Company expects it to be concluded in the first quarter of Biotec will be thorough in the process of identifying a US partner, in order to ensure the best possible setup. In parallel, Biotec BetaGlucans will focus on developing new-, and securing existing supplier agreements within animal health and nutrition, as well as pursuing opportunities within the field of cancer. In the enzyme market, ArcticZymes has a strong product offering, in addition to valuable longterm relationships with key customers. Further development of the Company s partnerships in molecular, diagnostic and adjacent markets, should enable ArcticZymes to increase its market share going forward.
11 Financial statement 2nd quarter 2016 INCOME STATEMENT - THE GROUP Q2 Jan - June (Amounts in NOK exept EPS) Sales Cost of goods sold Gross profit Other revenues Sum other revenues Personell expenses Other expenses EBITDA Depreciation and amortization expenses EBIT Finanical income, net EBT Tax Earnings after tax Basic EPS (profit for the period) -0,05-0,03-0,13-0,10 Diluted EPS (profit for the period) -0,05-0,03-0,13-0,10 BALANCE SHEET - THE GROUP (Amounts in NOK 1.000) Non-current assets Machinery and equipment Intangible assets Other financial assets Total non-current assets Current assets Inventories Trade receivables and other receivables Cash and cash equivalents Total current assets Total assets Equity Share capital Share premium capital Other equity Non-controlling interests Total equity Current liabilities Trade-, short term-, and other payables Total current liabilities Total equity and liabilities
12 CHANGES IN EQUITY - THE GROUP (Amounts in NOK 1000) Share capital Share premium capital Own shares Minority interests Other reserves Total equity Balance at Total comprehensive income/-loss for the period Transactions with shareholders: Employee stock option provision Purchase of own shares Sale of own shares Total transactions with shareholders Balance at Total comprehensive income/-loss for the period Transactions with shareholders: Employee stock option provision Total transactions with shareholders Balance at Total comprehensive income/-loss for the period Transactions with shareholders: Employee stock option provision Total transactions with shareholders Balance at CASH FLOW ANALYSIS - THE GROUP Q2 Jan - June (Amounts in NOK 1.000) Cash flow from operating activities: Profit after tax Adjustment: Depreciation Amortization Employee stock options Changes in working capital Inventory Account receivables and other receivables Payables and other current liabilities Net cash flow from operating activities Cash flow from investing activities: Purchase of fixed assets Change in long term receivables Net cash flow from investing activities Cash flow from financing activities: Cashflow Sale of own from shares private placement Net cash flow from financing activities Changes in cash and cash equivalents Cash and cash equivalents at the beginning of period Cash and cash equivalents at end of period
13 Notes to the interim accounts for 2nd quarter 2016 Note 1 - Basis of preparation of financial statements These financial statements are the unaudited interim consolidated financial statements (hereafter the Interim Financial Statements ) of Biotec Pharmacon ASA and its subsidiaries (hereafter the Group ) for the period ended June The Interim Financial Statements are prepared in accordance with the International Accounting Standard 34 (IAS 34). These Interim Financial Statements should be read in conjunction with the Consolidated Financial Statements for the year, ended December (hereafter the Annual Financial Statements ), as they provide an update of previously reported information. The accounting policies used in the Interim Financial Statements are consistent with those used in the Annual Financial Statements. The presentation of the Interim Financial Statements is consistent with the Annual Financial Statements. Where necessary, the comparatives have been reclassified or extended from the previously reported Interim Financial Statements to t ake into account any presentational changes made in the Annual Financial Statements or in these Interim Financial Statements. Income tax expense or benefit is recognized based upon the best estimate of the weighted average income tax rate expected for the full financial year. Deferred tax asset is accounted at NOK 0 in the balance sheet. Note 2 - Analysis of operating revenue and -expenses, segment information Services provided by the parent company are expensed at both segments according to agreements with actual subsidiary. Corporate overhead costs remain unallocated. Q2 Jan - June (Amounts in NOK 1.000) Sales revenue: Beta-Glucans Enzymes Group operating sales revenues Gross profit Beta-Glucans Enzymes Group gross profit Other revenues Beta-Glucans Enzymes Unallocated revenues corporate level Group other revenues Operating expenses: Beta-Glucans Enzymes Unallocated corporate expenses Group operating expenses EBITDA Beta-Glucans Enzymes Unallocated corporate expenses EBITDA Amortization: Beta-Glucans Enzymes Unallocated corporate expenses Group amortization EBIT Beta-Glucans Enzymes Unallocated corpoate expenses EBIT Oslo, August 16, 2016 The Board of Directors of Biotec Pharmacon ASA Erik Thorsen Olav Flaten Inger Rydin Chairman Director Director Richard Godfrey Masha Strømme Gerd Nilsen Svein W. F. Lien Director Director Director CEO
INTERIM REPORT Q3 AND 9M 2014
 INTERIM REPORT Q3 AND 9M 2014 THIRD QUARTER HIGHLIGHTS International market evaluation for Woulgan Biogel by Smith & Nephew continues at several sites in UK and Germany Woulgan Biogel survey ongoing in
INTERIM REPORT Q3 AND 9M 2014 THIRD QUARTER HIGHLIGHTS International market evaluation for Woulgan Biogel by Smith & Nephew continues at several sites in UK and Germany Woulgan Biogel survey ongoing in
INTERIM REPORT 1 st QUARTER 2012
 INTERIM REPORT 1 st QUARTER 2012 ENZYMES ArcticZymes Revenue still low primarily due to continued stock-reduction of SAP at Affymetrix Further broadening of the customer base for both the SAP and Cod UNG
INTERIM REPORT 1 st QUARTER 2012 ENZYMES ArcticZymes Revenue still low primarily due to continued stock-reduction of SAP at Affymetrix Further broadening of the customer base for both the SAP and Cod UNG
FIRST QUARTER REPORT 2006
 FIRST QUARTER REPORT 2006 Highlights The inclusion of patients for the phase II trial (Archangelsk and St. Petersburg, Russia) for treatment of diabetic ulcers has been almost completed. The clinical phase
FIRST QUARTER REPORT 2006 Highlights The inclusion of patients for the phase II trial (Archangelsk and St. Petersburg, Russia) for treatment of diabetic ulcers has been almost completed. The clinical phase
Q First quarter 2018
 Q1 2018 First quarter 2018 Highlights for the first quarter of 2018 o Group sales were NOK 14.2 million in the first quarter of 2018, compared to NOK 18.2 million in the first quarter of 2017, due to lower
Q1 2018 First quarter 2018 Highlights for the first quarter of 2018 o Group sales were NOK 14.2 million in the first quarter of 2018, compared to NOK 18.2 million in the first quarter of 2017, due to lower
ANNUAL REPORT Biotec Pharmacon ASA
 ANNUAL REPORT 2017 Index Board of Directors report 2017 05 Consolidated financial statements 21 Notes to the financial statements for 2017 27 Financial statements parent company 53 Notes to the financial
ANNUAL REPORT 2017 Index Board of Directors report 2017 05 Consolidated financial statements 21 Notes to the financial statements for 2017 27 Financial statements parent company 53 Notes to the financial
THIRD QUARTER REPORT 2005
 THIRD QUARTER REPORT 2005 Highlights In September the US Food and Drug Administration approved the application for the first clinical trial with SBG in combination with a monoclonal cancer antibody. Patient
THIRD QUARTER REPORT 2005 Highlights In September the US Food and Drug Administration approved the application for the first clinical trial with SBG in combination with a monoclonal cancer antibody. Patient
Q Second quarter 2018
 Q2 2018 Second quarter 2018 Highlights for the second quarter of 2018 o o o o o o Raised NOK 22.1 million in new equity by issuing 4,390,000 new shares. The capital increase was done as a private placement
Q2 2018 Second quarter 2018 Highlights for the second quarter of 2018 o o o o o o Raised NOK 22.1 million in new equity by issuing 4,390,000 new shares. The capital increase was done as a private placement
SECOND QUARTER REPORT 2006
 SECOND QUARTER REPORT 2006 Highlights Treatment of patients in the phase II diabetic ulcers trial (Archangelsk and St. Petersburg, Russia) has been completed and results from the trial are expected in
SECOND QUARTER REPORT 2006 Highlights Treatment of patients in the phase II diabetic ulcers trial (Archangelsk and St. Petersburg, Russia) has been completed and results from the trial are expected in
30 MAY First quarter 2018 PRESENTED BY TOM RÖNNLUND, CEO Q1 2018
 30 MAY 2018 First quarter 2018 PRESENTED BY TOM RÖNNLUND, CEO Q1 2018 Q1 Summary Navamedic reported revenues of NOK 42.6 million, compared to NOK 78.2 in Q1 2017 The Q1 2018 revenues decreased by 4.0 %
30 MAY 2018 First quarter 2018 PRESENTED BY TOM RÖNNLUND, CEO Q1 2018 Q1 Summary Navamedic reported revenues of NOK 42.6 million, compared to NOK 78.2 in Q1 2017 The Q1 2018 revenues decreased by 4.0 %
INTERIM REPORT - Q3 2009
 INTERIM REPORT - Q3 2009 FOR EARLIER DISEASE DETECTION 2 Highlights Norwegian Research Council Names DiaGenic Most Innovative Company of the Year Highlights Scientific marketing of ADtect and BCtect started
INTERIM REPORT - Q3 2009 FOR EARLIER DISEASE DETECTION 2 Highlights Norwegian Research Council Names DiaGenic Most Innovative Company of the Year Highlights Scientific marketing of ADtect and BCtect started
PROSPECTUS. Biotec Pharmacon ASA (A public limited liability company organised under the laws of Norway)
 PROSPECTUS Biotec Pharmacon ASA (A public limited liability company organised under the laws of Norway) LISTING ON OSLO STOCK EXCHANGE OF 9,500,000 NEW SHARES IN BIOTEC PHARMACON ASA ISSUED IN CONNECTION
PROSPECTUS Biotec Pharmacon ASA (A public limited liability company organised under the laws of Norway) LISTING ON OSLO STOCK EXCHANGE OF 9,500,000 NEW SHARES IN BIOTEC PHARMACON ASA ISSUED IN CONNECTION
DiaGenic ASA Interim Report Q for early disease detection
 DiaGenic ASA Interim Report Q1 2010 for early disease detection Growing market attention; Molecular diagnostics and biomarkers for Pharma HIGHLIGHTS >> Distribution agreement with Ferrer on ADtect >> First
DiaGenic ASA Interim Report Q1 2010 for early disease detection Growing market attention; Molecular diagnostics and biomarkers for Pharma HIGHLIGHTS >> Distribution agreement with Ferrer on ADtect >> First
INTERIM REPORT - Q1 2009
 INTERIM REPORT - Q1 2009 FOR EARLIER DISEASE DETECTION 2 Highlights A quarter dedicated to progress on CE marking Highlights Finalized the necessary analytical work for CE marking of both BCtect and ADtect
INTERIM REPORT - Q1 2009 FOR EARLIER DISEASE DETECTION 2 Highlights A quarter dedicated to progress on CE marking Highlights Finalized the necessary analytical work for CE marking of both BCtect and ADtect
Interim Report Q1 2013
 Interim Report Q1 2013 Private placement raising NOK 30 million completed, full focus on Alzheimer s disease product portfolio development Highlights Highlights ÂÂ ÂÂ On 7 March 2013 DiaGenic raised NOK
Interim Report Q1 2013 Private placement raising NOK 30 million completed, full focus on Alzheimer s disease product portfolio development Highlights Highlights ÂÂ ÂÂ On 7 March 2013 DiaGenic raised NOK
Photocure ASA - Third Quarter Report 2008
 Photocure ASA - Third Quarter Report 2008 Highlights Sales revenues increase 52% to NOK 23.6 million ( from NOK 15.6 million in third quarter 2007) Operating profit improved by NOK 13.7 million to NOK
Photocure ASA - Third Quarter Report 2008 Highlights Sales revenues increase 52% to NOK 23.6 million ( from NOK 15.6 million in third quarter 2007) Operating profit improved by NOK 13.7 million to NOK
Photocure ASA. The world leader in photodynamic technology. Presentation of second quarter and first half year 2011 results
 Brilliance in photodynamic technology Photocure ASA The world leader in photodynamic technology Presentation of second quarter and first half year 2011 results 18 August 2011 Kjetil Hestdal, President
Brilliance in photodynamic technology Photocure ASA The world leader in photodynamic technology Presentation of second quarter and first half year 2011 results 18 August 2011 Kjetil Hestdal, President
Norlandia Health & Care Group AS Q3 Interim Report 2017
 Norlandia Health & Care Group AS Q3 Interim Report 2017 CONTENT CONTENT... 2 KEY FIGURES... 3 Q3 2017 HIGHLIGHTS... 3 NORLANDIA HEALTH & CARE GROUP AS... 5 GROUP ACTIVITIES... 5 FINANCIALS... 6 GROUP FINANCIAL
Norlandia Health & Care Group AS Q3 Interim Report 2017 CONTENT CONTENT... 2 KEY FIGURES... 3 Q3 2017 HIGHLIGHTS... 3 NORLANDIA HEALTH & CARE GROUP AS... 5 GROUP ACTIVITIES... 5 FINANCIALS... 6 GROUP FINANCIAL
Brilliance in photodynamic technology TM. Establishing a Specialty Pharma company
 Brilliance in photodynamic technology TM Establishing a Specialty Pharma company 28 April 2010 Results for the 1 st quarter 2010 Highlights for the first quarter 2010 (2009 figures in brackets) Hexvix
Brilliance in photodynamic technology TM Establishing a Specialty Pharma company 28 April 2010 Results for the 1 st quarter 2010 Highlights for the first quarter 2010 (2009 figures in brackets) Hexvix
PHOTOCURE ASA RESULTS FOR FIRST QUARTER MAY Kjetil Hestdal, MD, President & CEO Erik Dahl, CFO
 PHOTOCURE ASA RESULTS FOR FIRST QUARTER 2018 23 MAY 2018 Kjetil Hestdal, MD, President & CEO Erik Dahl, CFO DISCLAIMER The information included in this Presentation contains certain forward-looking statements
PHOTOCURE ASA RESULTS FOR FIRST QUARTER 2018 23 MAY 2018 Kjetil Hestdal, MD, President & CEO Erik Dahl, CFO DISCLAIMER The information included in this Presentation contains certain forward-looking statements
Interim Report, First Quarter 2014
 Interim Report, First Quarter 2014 CORTENDO REPORTS RESULTS AND ACTIVITIES FOR THE FIRST QUARTER 2014 FIRST AND POST QUARTER HIGHLIGHTS Continued progress on the start-up of NormoCort Phase 3 trial While
Interim Report, First Quarter 2014 CORTENDO REPORTS RESULTS AND ACTIVITIES FOR THE FIRST QUARTER 2014 FIRST AND POST QUARTER HIGHLIGHTS Continued progress on the start-up of NormoCort Phase 3 trial While
Photocure ASA. Evolving into a Specialty Pharma company. Results for the fourth quarter and full year 2011
 Brilliance in photodynamic technology Photocure ASA Evolving into a Specialty Pharma company Results for the fourth quarter and full year 2011 16 February 2012 Kjetil Hestdal, President & CEO Torbjørn
Brilliance in photodynamic technology Photocure ASA Evolving into a Specialty Pharma company Results for the fourth quarter and full year 2011 16 February 2012 Kjetil Hestdal, President & CEO Torbjørn
FOURTH QUARTER Highlights from fourth quarter 2006 include: Strong cash flow from operations of 254 MNOK (131 MNOK in fourth quarter 2005)
 FOURTH QUARTER 2006 Highlights from fourth quarter 2006 include: Revenues of 1,054 MNOK (+56 percent relative to 675 MNOK in fourth quarter 2005) Operating profit of 135 MNOK (79 MNOK in fourth quarter
FOURTH QUARTER 2006 Highlights from fourth quarter 2006 include: Revenues of 1,054 MNOK (+56 percent relative to 675 MNOK in fourth quarter 2005) Operating profit of 135 MNOK (79 MNOK in fourth quarter
Second Quarter July 28 th, Tom Rönnlund, CEO
 Second Quarter 2017 July 28 th, 2017 Tom Rönnlund, CEO AGENDA Agenda Q2 highlights and key figures Pharma & Healthcare commercial update Medtech commercial update Financials Outlook Navamedic 2 Q1 HIGHLIGHTS
Second Quarter 2017 July 28 th, 2017 Tom Rönnlund, CEO AGENDA Agenda Q2 highlights and key figures Pharma & Healthcare commercial update Medtech commercial update Financials Outlook Navamedic 2 Q1 HIGHLIGHTS
Third consecutive profitable quarter with continued strong growth
 Interim report for the period 1 January 30 September 2015 Announcement No. 10/2015 To NASDAQ Copenhagen Exiqon A/S Skelstedet 16 2950 Vedbæk Denmark 26 October 2015 Phone: +45 4566 0888 Fax: +45 4566 1888
Interim report for the period 1 January 30 September 2015 Announcement No. 10/2015 To NASDAQ Copenhagen Exiqon A/S Skelstedet 16 2950 Vedbæk Denmark 26 October 2015 Phone: +45 4566 0888 Fax: +45 4566 1888
The world leader in photodynamic technology
 Brilliance in photodynamic technology Results for the first quarter 2011 The world leader in photodynamic technology 27 April 2011 Highlights for the first quarter 2011 (Q1 2010 figures in brackets) Hexvix
Brilliance in photodynamic technology Results for the first quarter 2011 The world leader in photodynamic technology 27 April 2011 Highlights for the first quarter 2011 (Q1 2010 figures in brackets) Hexvix
PHOTOCURE ASA RESULTS FOR FOURTH QUARTER AND FULL YEAR FEBRUARY Kjetil Hestdal, MD, President & CEO Erik Dahl, CFO
 PHOTOCURE ASA RESULTS FOR FOURTH QUARTER AND FULL YEAR 2017 27 FEBRUARY 2018 Kjetil Hestdal, MD, President & CEO Erik Dahl, CFO DISCLAIMER The information included in this Presentation contains certain
PHOTOCURE ASA RESULTS FOR FOURTH QUARTER AND FULL YEAR 2017 27 FEBRUARY 2018 Kjetil Hestdal, MD, President & CEO Erik Dahl, CFO DISCLAIMER The information included in this Presentation contains certain
THIRD QUARTER Strong performance in Collection Technology Deposit. Improved performance and outlook in Industrial Processing Technology
 THIRD QUARTER 2009 Highlights from third quarter 2009 include: Strong performance in Collection Technology Deposit Improved performance and outlook in Industrial Processing Technology California negatively
THIRD QUARTER 2009 Highlights from third quarter 2009 include: Strong performance in Collection Technology Deposit Improved performance and outlook in Industrial Processing Technology California negatively
2008 REPORT. Agreement on the breast cancer test signed with SRL Ranbaxy. Indian multi-centre breast cancer study completed
 Q2 28 REPORT 1ST HALF-YEAR HIGHLIGHTS: Agreement on the breast cancer test signed with SRL Ranbaxy Indian multi-centre breast cancer study completed NOK 44.8 million share issue European patent granted
Q2 28 REPORT 1ST HALF-YEAR HIGHLIGHTS: Agreement on the breast cancer test signed with SRL Ranbaxy Indian multi-centre breast cancer study completed NOK 44.8 million share issue European patent granted
Q May 10, Tom Rönnlund, CEO
 Q1 2017 May 10, 2017 Tom Rönnlund, CEO AGENDA Agenda Q1 highlights and financials Pharma & Healthcare commercial update Medtech commercial update Outlook 10.05.2017 Navamedic 2 Q1 HIGHLIGHTS Q1 Highlights
Q1 2017 May 10, 2017 Tom Rönnlund, CEO AGENDA Agenda Q1 highlights and financials Pharma & Healthcare commercial update Medtech commercial update Outlook 10.05.2017 Navamedic 2 Q1 HIGHLIGHTS Q1 Highlights
PHOTOCURE ASA BUILDING A SPECIALTY PHARMA COMPANY
 PHOTOCURE ASA BUILDING A SPECIALTY PHARMA COMPANY RESULTS OF THE SECOND QUARTER 2013 22 August 2013 Kjetil Hestdal, MD, President & CEO Erik Dahl, CFO DISCLAIMER The information included in this Presentation
PHOTOCURE ASA BUILDING A SPECIALTY PHARMA COMPANY RESULTS OF THE SECOND QUARTER 2013 22 August 2013 Kjetil Hestdal, MD, President & CEO Erik Dahl, CFO DISCLAIMER The information included in this Presentation
Brilliance in photodynamic technology TM. Second quarter August 16th, 2007
 Brilliance in photodynamic technology TM Second quarter 2007 August 16th, 2007 Total revenues increased 49% to MNOK 29.1 (19.5) Hexvix commercialisation gaining momentum Milestones payments of Euro 1.0
Brilliance in photodynamic technology TM Second quarter 2007 August 16th, 2007 Total revenues increased 49% to MNOK 29.1 (19.5) Hexvix commercialisation gaining momentum Milestones payments of Euro 1.0
INTERIM REPORT 1 JANUARY 31 MARCH 2018
 INTERIM REPORT 1 JANUARY 31 MARCH 2018 Growth continues 1 JANUARY 31 MARCH 2018 (3 MONTHS) Net sales rose by 4 percent to SEK 597 million (576). EBITA rose by 7 percent to SEK 57 million (54), corresponding
INTERIM REPORT 1 JANUARY 31 MARCH 2018 Growth continues 1 JANUARY 31 MARCH 2018 (3 MONTHS) Net sales rose by 4 percent to SEK 597 million (576). EBITA rose by 7 percent to SEK 57 million (54), corresponding
NEXT Biometrics Group ASA
 NEXT Biometrics Group ASA Quarterly report Q1 2017 Highlights Revenue of NOK 24.1 million vs NOK 5.2 million Q1-16 and in Q1-17 vs NOK 31.8 million in Q4-16 Accumulated shipments pass 2.0 million sensors
NEXT Biometrics Group ASA Quarterly report Q1 2017 Highlights Revenue of NOK 24.1 million vs NOK 5.2 million Q1-16 and in Q1-17 vs NOK 31.8 million in Q4-16 Accumulated shipments pass 2.0 million sensors
Interim report May July 2014/15
 August 28, 2014 Interim report May July 2014/15 Order bookings increased 12* percent to SEK 2,341 M (2,027). Net sales decreased 4* percent to SEK 1,865 M (1,912). EBITA amounted to SEK -38 M (148) before
August 28, 2014 Interim report May July 2014/15 Order bookings increased 12* percent to SEK 2,341 M (2,027). Net sales decreased 4* percent to SEK 1,865 M (1,912). EBITA amounted to SEK -38 M (148) before
RAYSEARCH LABORATORIES AB (PUBL)
 RAYSEARCH LABORATORIES AB (PUBL) INTERIM REPORT JANUARY 1 SEPTEMBER 30, 2014 JANUARY 1 SEPTEMBER 30, 2014 Net sales for the period amounted to SEK 177.4 M (114.4) Profit after tax was SEK 19.1 M (loss:
RAYSEARCH LABORATORIES AB (PUBL) INTERIM REPORT JANUARY 1 SEPTEMBER 30, 2014 JANUARY 1 SEPTEMBER 30, 2014 Net sales for the period amounted to SEK 177.4 M (114.4) Profit after tax was SEK 19.1 M (loss:
Year-end report 1 APRIL MARCH 2016
 Year-end report 1 APRIL 2015-31 MARCH 2016 1 January 2016 31 March 2016 (3 months) Net sales in the fourth quarter rose by 59 percent to SEK 452.7 million (284.7), of which organic growth totalled 6 percent.
Year-end report 1 APRIL 2015-31 MARCH 2016 1 January 2016 31 March 2016 (3 months) Net sales in the fourth quarter rose by 59 percent to SEK 452.7 million (284.7), of which organic growth totalled 6 percent.
Q3/17. Third Quarter 2017 Report
 Q3/17 Third Quarter 2017 Report Highlights for the third quarter Navamedic reported revenues of NOK 44.9 million in third quarter 2017, down from NOK 65.2 million in the same period in 2016, following
Q3/17 Third Quarter 2017 Report Highlights for the third quarter Navamedic reported revenues of NOK 44.9 million in third quarter 2017, down from NOK 65.2 million in the same period in 2016, following
AMINO TECHNOLOGIES PLC INTERIM RESULTS FOR THE SIX MONTHS ENDED 31 MAY 2014 STRONG OPERATING PROFIT AND CASH GENERATION
 AMINO TECHNOLOGIES PLC INTERIM RESULTS FOR THE SIX MONTHS ENDED 31 MAY 2014 STRONG OPERATING PROFIT AND CASH GENERATION Amino Technologies plc ('Amino' or the 'Company') (LSE: AMO), the Cambridge-based
AMINO TECHNOLOGIES PLC INTERIM RESULTS FOR THE SIX MONTHS ENDED 31 MAY 2014 STRONG OPERATING PROFIT AND CASH GENERATION Amino Technologies plc ('Amino' or the 'Company') (LSE: AMO), the Cambridge-based
Interim Report. First Quarter 2018, BioPorto Group. May 3, 2018 Announcement no. 10. BioPorto A/S CVR DK
 Interim Report First Quarter 2018, BioPorto Group May 3, 2018 Announcement no. 10 BioPorto A/S CVR DK-17500317 Highlights Important milestones in global rollout of The NGAL Test secured in first quarter
Interim Report First Quarter 2018, BioPorto Group May 3, 2018 Announcement no. 10 BioPorto A/S CVR DK-17500317 Highlights Important milestones in global rollout of The NGAL Test secured in first quarter
Other products and technologies are currently in the research and development or pre-commercial stage. These technologies include:
 The MD&A provides commentary on the results of operations for the six months ended June 30, 2012 and 2011, the financial position as at June 30, 2012, and the outlook of Ceapro Inc. ( Ceapro ) based on
The MD&A provides commentary on the results of operations for the six months ended June 30, 2012 and 2011, the financial position as at June 30, 2012, and the outlook of Ceapro Inc. ( Ceapro ) based on
Page 2. 1 st quarter 2015
 Page 2 1 st quarter 2015 The 1 st quarter is normally the weakest quarter. Winter entails a low level of production, and delayed start-up in the spring results in additional costs being incurred. Adjusted
Page 2 1 st quarter 2015 The 1 st quarter is normally the weakest quarter. Winter entails a low level of production, and delayed start-up in the spring results in additional costs being incurred. Adjusted
Brilliance in photodynamic technology TM. - from Biotech to Specialty Pharma
 Brilliance in photodynamic technology TM - from Biotech to Specialty Pharma 19 February 2010 Results for the 4 th quarter and full year 2009 Highlights for the fourth quarter 2009 (2008 figures in brackets)
Brilliance in photodynamic technology TM - from Biotech to Specialty Pharma 19 February 2010 Results for the 4 th quarter and full year 2009 Highlights for the fourth quarter 2009 (2008 figures in brackets)
Results for the fourth quarter and full year Establishing a Specialty Pharma company
 Results for the fourth quarter and full year 2011 Establishing a Specialty Pharma company 16 February 2012 Highlights for the fourth quarter and full year 2011 Photocure (OSE: PHO), a Norwegian specialty
Results for the fourth quarter and full year 2011 Establishing a Specialty Pharma company 16 February 2012 Highlights for the fourth quarter and full year 2011 Photocure (OSE: PHO), a Norwegian specialty
Viking Redningstjeneste Topco AS. Interim financial statements 4Q 2018
 Viking Redningstjeneste Topco AS Interim financial statements 4Q 2018 Quarterly report October December 2018 Viking Redningstjeneste Topco AS Fourth quarter 2018 Org no. 998 858 690 Quarterly report FOURTH
Viking Redningstjeneste Topco AS Interim financial statements 4Q 2018 Quarterly report October December 2018 Viking Redningstjeneste Topco AS Fourth quarter 2018 Org no. 998 858 690 Quarterly report FOURTH
Clavis Pharma ASA. First Quarter Report 2008
 Clavis Pharma ASA First Quarter Report 2008 Clavis Pharma uses its proprietary Lipid Vector Technology (LVT) to develop new and superior pharmaceuticals by improving already established drugs. The Company
Clavis Pharma ASA First Quarter Report 2008 Clavis Pharma uses its proprietary Lipid Vector Technology (LVT) to develop new and superior pharmaceuticals by improving already established drugs. The Company
INTERIM REPORT Q XXL ASA HIGHLIGHTS. Q2 Growth
 INTERIM REPORT Q2 2014 XXL ASA HIGHLIGHTS Total revenues of NOK 1 246 million (NOK 945 million), up 32 per cent EBITDA increased by 47 per cent to NOK 184 million Successful opening in Finland One new
INTERIM REPORT Q2 2014 XXL ASA HIGHLIGHTS Total revenues of NOK 1 246 million (NOK 945 million), up 32 per cent EBITDA increased by 47 per cent to NOK 184 million Successful opening in Finland One new
FORWARD-LOOKING STATEMENTS
 of Financial Condition and Results of Operations of Profound Medical Corp. for the Three and Six Months Ended June 30, 2017 The following ( MD&A ) prepared as of August 24, 2017 should be read in conjunction
of Financial Condition and Results of Operations of Profound Medical Corp. for the Three and Six Months Ended June 30, 2017 The following ( MD&A ) prepared as of August 24, 2017 should be read in conjunction
ASPOCOMP S HALF YEAR FINANCIAL REPORT 2016
 ASPOCOMP S HALF YEAR FINANCIAL REPORT 2016 Key figures 4-6/2016 in brief 4-6/2016 4-6/2015 Change Net sales 5.3 M 4.4 M 1.0 M EBITDA 0.4 M -0.2 M 0.6 M Comparable operating result 0.2 M -0.3 M 0.5 M %
ASPOCOMP S HALF YEAR FINANCIAL REPORT 2016 Key figures 4-6/2016 in brief 4-6/2016 4-6/2015 Change Net sales 5.3 M 4.4 M 1.0 M EBITDA 0.4 M -0.2 M 0.6 M Comparable operating result 0.2 M -0.3 M 0.5 M %
Interim Report. First half of 2017, BioPorto Group. August 10, 2017 Announcement no. 10. BioPorto A/S CVR DK
 Interim Report First half of 2017, BioPorto Group August 10, 2017 Announcement no. 10 BioPorto A/S CVR DK-17500317 Highlights US Clinical study commencing according to announced plan BioPorto commenced
Interim Report First half of 2017, BioPorto Group August 10, 2017 Announcement no. 10 BioPorto A/S CVR DK-17500317 Highlights US Clinical study commencing according to announced plan BioPorto commenced
Vision, Core Business, and Strategy
 The MD&A provides commentary on the results of operations for the periods ended March 31, 2011 and 2010, the financial position as at March 31, 2011 and the outlook of Ceapro Inc. ( Ceapro ) based on information
The MD&A provides commentary on the results of operations for the periods ended March 31, 2011 and 2010, the financial position as at March 31, 2011 and the outlook of Ceapro Inc. ( Ceapro ) based on information
INTERIM REPORT 1 JANUARY 30 JUNE 2018
 INTERIM REPORT 1 JANUARY 30 JUNE 2018 Continued favourable development 1 APRIL 30 JUNE 2018 (3 MONTHS) Net sales increased by 9 percent to SEK 622 million (572). EBITA increased by 9 percent to SEK 63
INTERIM REPORT 1 JANUARY 30 JUNE 2018 Continued favourable development 1 APRIL 30 JUNE 2018 (3 MONTHS) Net sales increased by 9 percent to SEK 622 million (572). EBITA increased by 9 percent to SEK 63
INTERIM REPORT Q3 JANUARY-SEPTEMBER 2017 SEDANA MEDICAL AB (PUBL) Q1 Q2
 INTERIM REPORT JANUARY-SEPTEMBER 2017 SEDANA MEDICAL AB (PUBL) Q1 Q2 Q4 SEDANA MEDICAL, INTERIM REPORT, JANUARY SEPTEMBER 2017 Financial summary July-September Net sales during the third quarter amounted
INTERIM REPORT JANUARY-SEPTEMBER 2017 SEDANA MEDICAL AB (PUBL) Q1 Q2 Q4 SEDANA MEDICAL, INTERIM REPORT, JANUARY SEPTEMBER 2017 Financial summary July-September Net sales during the third quarter amounted
Interim Results for the six months ended 31 July 2013
 1 October LIDCO GROUP PLC ( LiDCO or the Company ) Interim Results for the six months LiDCO (AIM:LID), the hemodynamic monitoring Company, today announces its Interim Results for the six months, which
1 October LIDCO GROUP PLC ( LiDCO or the Company ) Interim Results for the six months LiDCO (AIM:LID), the hemodynamic monitoring Company, today announces its Interim Results for the six months, which
Viking Redningstjeneste Topco AS. Interim financial statements 1Q 2018
 Viking Redningstjeneste Topco AS Interim financial statements 1Q 2018 Quarterly report January - March 2018 Viking Redningstjeneste Topco AS Org no. 998 858 690 First quarter 2018 Quarterly report FIRST
Viking Redningstjeneste Topco AS Interim financial statements 1Q 2018 Quarterly report January - March 2018 Viking Redningstjeneste Topco AS Org no. 998 858 690 First quarter 2018 Quarterly report FIRST
Redwood Pharma AB (publ) Interim Report January June 2018 SPOTLIGHT STOCK MARKET: REDW REDWOODPHARMA.COM. RedwoodPharma
 Redwood Pharma AB (publ) Interim Report January June 2018 SPOTLIGHT STOCK MARKET: REDW REDWOODPHARMA.COM RedwoodPharma Redwood Pharma AB (publ) Interim Report January June 2018 The Period January 1 June
Redwood Pharma AB (publ) Interim Report January June 2018 SPOTLIGHT STOCK MARKET: REDW REDWOODPHARMA.COM RedwoodPharma Redwood Pharma AB (publ) Interim Report January June 2018 The Period January 1 June
Half-Year Report 2005
 Half-Year Report 2005 SHL TeleMedicine Ltd. 1 January - 30 June 1 Dear Shareholders, SHL s first half of 2005 marked by signing of significant telemedicine contracts in Germany and US and divesture of
Half-Year Report 2005 SHL TeleMedicine Ltd. 1 January - 30 June 1 Dear Shareholders, SHL s first half of 2005 marked by signing of significant telemedicine contracts in Germany and US and divesture of
P R E S S R E L E A S E
 P R E S S R E L E A S E from ASSA ABLOY AB (publ) 6 February 2003 No. 03/03 REPORT FOR THE FOURTH QUARTER OF 2002 (YEAR-END REPORT) Sales increased 3% for the quarter, 12% in local currencies, 2% organic
P R E S S R E L E A S E from ASSA ABLOY AB (publ) 6 February 2003 No. 03/03 REPORT FOR THE FOURTH QUARTER OF 2002 (YEAR-END REPORT) Sales increased 3% for the quarter, 12% in local currencies, 2% organic
Interim report Q4 2018
 Interim report Q4 2018 Interim report Q4 2018 Kid ASA Dear Shareholders The fourth quarter of 2018 was the best three month period ever for Kid. The early winter and Christmas season is extremely busy
Interim report Q4 2018 Interim report Q4 2018 Kid ASA Dear Shareholders The fourth quarter of 2018 was the best three month period ever for Kid. The early winter and Christmas season is extremely busy
TruScreen Interim Report
 2018 INTERIM REPORT TruScreen Interim Report 2018 1 CONTENTS Our Vision 03 Progress Against Strategic Goals 04 Half Year Results Snapshot 05 Chairman and CEO s Report 06 Interim Financial Statements 08
2018 INTERIM REPORT TruScreen Interim Report 2018 1 CONTENTS Our Vision 03 Progress Against Strategic Goals 04 Half Year Results Snapshot 05 Chairman and CEO s Report 06 Interim Financial Statements 08
Four new launches of in-licensed products this quarter in addition to the 5 new products earlier launched in 2018.
 INTERIM REPORT JANUARY SEPTEMBER 2018 Net sales amounted to SEK 263.3 (237.2) million EBITDA was SEK 15.6 (-2.3) million Basic earnings per share were SEK -0.17 (-0.32) JULY SEPTEMBER 2018 Net sales amounted
INTERIM REPORT JANUARY SEPTEMBER 2018 Net sales amounted to SEK 263.3 (237.2) million EBITDA was SEK 15.6 (-2.3) million Basic earnings per share were SEK -0.17 (-0.32) JULY SEPTEMBER 2018 Net sales amounted
Exiqon A/S (NASDAQ OMX Copenhagen: EXQ ) today announced results for the first three months of 2015:
 Interim report for the quarter 1 January 31 March 2015 Announcement No. 5/2015 To NASDAQ OMX Copenhagen A/S 7 May 2015 Exiqon A/S Skelstedet 16 2950 Vedbæk Denmark CVR nr. 18 98 44 31 Phone: +45 4566 0888
Interim report for the quarter 1 January 31 March 2015 Announcement No. 5/2015 To NASDAQ OMX Copenhagen A/S 7 May 2015 Exiqon A/S Skelstedet 16 2950 Vedbæk Denmark CVR nr. 18 98 44 31 Phone: +45 4566 0888
FOURTH QUARTER Solid performance in Collection Technology. Continued improved performance and order inflow in Industrial Processing Technology
 FOURTH QUARTER 2009 Highlights from fourth quarter 2009: Solid performance in Collection Continued improved performance and order inflow in Industrial Processing California adversely affected by reduced
FOURTH QUARTER 2009 Highlights from fourth quarter 2009: Solid performance in Collection Continued improved performance and order inflow in Industrial Processing California adversely affected by reduced
REPORT 1ST QUARTER NRC GROUP ASA / Q1 REPORT 2018
 REPORT 1ST QUARTER 2018 NRC GROUP ASA / Q1 REPORT 2018 Highlights 1 st quarter 2018 / KEY EVENTS Record-high order intake of NOK 1,727 million, an increase of 126% compared to 1 st quarter 2017 Appointed
REPORT 1ST QUARTER 2018 NRC GROUP ASA / Q1 REPORT 2018 Highlights 1 st quarter 2018 / KEY EVENTS Record-high order intake of NOK 1,727 million, an increase of 126% compared to 1 st quarter 2017 Appointed
Financial Report Q4 2017
 Financial Report Q4 2017 Overview Bond issuer Care Bidco AS Unicare group Unicare is one of the largest private healthcare and care service providers in Norway. The Company was founded in 2008 and is now
Financial Report Q4 2017 Overview Bond issuer Care Bidco AS Unicare group Unicare is one of the largest private healthcare and care service providers in Norway. The Company was founded in 2008 and is now
HIGHLIGHTS INTERIM REPORT Q XXL ASA. Q1 Growth
 INTERIM REPORT Q1 2018 XXL ASA HIGHLIGHTS Total revenues of NOK 2 070 million (NOK 1 713 million), up 21 per cent E-commerce growth of 42 per cent EBITDA of NOK 51 million (NOK 34 million) Solid cash flow
INTERIM REPORT Q1 2018 XXL ASA HIGHLIGHTS Total revenues of NOK 2 070 million (NOK 1 713 million), up 21 per cent E-commerce growth of 42 per cent EBITDA of NOK 51 million (NOK 34 million) Solid cash flow
2.3% Interim Report. January March Good growth supported by successful launch and sales ramp-up in USA and Canada
 Q1 Interim Report January March Doro AB Corporate Identity Number 556161-9429 22.3% Net sales growth 2.3% EBIT margin Good growth supported by successful launch and sales ramp-up in USA and Canada January
Q1 Interim Report January March Doro AB Corporate Identity Number 556161-9429 22.3% Net sales growth 2.3% EBIT margin Good growth supported by successful launch and sales ramp-up in USA and Canada January
Bachem. Leading beyond peptides
 Bachem. Leading beyond peptides Half-Year Report 2007 Half-Year Report 2007 New record results for Bachem in the first half of 2007 Sales growth of 25.4% in CHF and 26.3% in local currencies APIs and new
Bachem. Leading beyond peptides Half-Year Report 2007 Half-Year Report 2007 New record results for Bachem in the first half of 2007 Sales growth of 25.4% in CHF and 26.3% in local currencies APIs and new
Q Q Q Q2 2017
 Financial Report - Results and financial position Report - Summary: Key Ratios Dignitana Group Q1- Q1- Full year Net revenues, TSEK 5 758 957 11 886 2 108 8 902 Total revenues TSEK 5 852 966 12 029 2 173
Financial Report - Results and financial position Report - Summary: Key Ratios Dignitana Group Q1- Q1- Full year Net revenues, TSEK 5 758 957 11 886 2 108 8 902 Total revenues TSEK 5 852 966 12 029 2 173
Interim Report Q4 2013
 Interim Report Q4 2013 Organizational downsizing and cost reductions implemented and the board has presented a proposal to secure financing of DiaGenic Highlights: Highlights 2 ÂÂ ÂÂ ÂÂ ÂÂ ÂÂ On 10th October
Interim Report Q4 2013 Organizational downsizing and cost reductions implemented and the board has presented a proposal to secure financing of DiaGenic Highlights: Highlights 2 ÂÂ ÂÂ ÂÂ ÂÂ ÂÂ On 10th October
Hematology is in our blood
 Hematology is in our blood Boule Diagnostics AB Company presentation, Introduce Investor Day December 4, 2018 Fredrik Dalborg, CEO and Group President 2018-12-04 BOULE DIAGNOSTICS (1) Copyright 2018, Boule
Hematology is in our blood Boule Diagnostics AB Company presentation, Introduce Investor Day December 4, 2018 Fredrik Dalborg, CEO and Group President 2018-12-04 BOULE DIAGNOSTICS (1) Copyright 2018, Boule
Interim report January September 2017
 Capio AB (publ) Interim report January September 2017 July September 2017 Net sales MSEK 3,455 (3,168). Organic sales growth 2.2% (2.6) and total sales growth 9.1% (3.7) EBITDA 1 MSEK 168 (200) and margin
Capio AB (publ) Interim report January September 2017 July September 2017 Net sales MSEK 3,455 (3,168). Organic sales growth 2.2% (2.6) and total sales growth 9.1% (3.7) EBITDA 1 MSEK 168 (200) and margin
MOLOGEN AG: Interim Financial Statements as of March 31, 2010
 MOLOGEN AG: Interim Financial Statements as of March 31, 2010 Content Foreword... Page 3 Interim management report for the period from January 1 to March 31, 2010... Page 5 Statement of financial position
MOLOGEN AG: Interim Financial Statements as of March 31, 2010 Content Foreword... Page 3 Interim management report for the period from January 1 to March 31, 2010... Page 5 Statement of financial position
Year-end report 2017 January - December YEAR-END REPORT 2017 OCTOBER DECEMBER 2017 JANUARY DECEMBER 2017
 Year-end report 2017 January - December Troax Group AB (publ) Hillerstorp 12th of February, 2018 YEAR-END REPORT 2017 OCTOBER DECEMBER 2017 Order intake increased by 17 per cent to 38,4 (32,8) MEUR. Adjusted
Year-end report 2017 January - December Troax Group AB (publ) Hillerstorp 12th of February, 2018 YEAR-END REPORT 2017 OCTOBER DECEMBER 2017 Order intake increased by 17 per cent to 38,4 (32,8) MEUR. Adjusted
Baird Global Healthcare Conference
 Baird Global Healthcare Conference Jason Meggs Chief Financial Officer September 6, 2018 Forward-Looking Statements, Non-GAAP Financial Measures, and Basis of Financial Presentation Forward-Looking Statements
Baird Global Healthcare Conference Jason Meggs Chief Financial Officer September 6, 2018 Forward-Looking Statements, Non-GAAP Financial Measures, and Basis of Financial Presentation Forward-Looking Statements
YEAR-END REPORT 2017 SEDANA MEDICAL AB (PUBL) Q1 Q2 Q3
 YEAR-END REPORT 2017 SEDANA MEDICAL AB (PUBL) Q1 Q2 Q3 Q4 SEDANA MEDICAL, YEAR-END REPORT 2017 Financial summary October December Net sales during the fourth quarter amounted to 10,795 (8,872) KSEK, corresponding
YEAR-END REPORT 2017 SEDANA MEDICAL AB (PUBL) Q1 Q2 Q3 Q4 SEDANA MEDICAL, YEAR-END REPORT 2017 Financial summary October December Net sales during the fourth quarter amounted to 10,795 (8,872) KSEK, corresponding
Cash flow from operations in the quarter of NOK 51.5 million
 Revenues of NOK 436.2 million, an increase of 5.1 %. EBITDA of NOK 46.1 million down from NOK 62.5 million. One-off costs for recruitment and severance of NOK 3.7 million taken in the quarter. EBITDA margin
Revenues of NOK 436.2 million, an increase of 5.1 %. EBITDA of NOK 46.1 million down from NOK 62.5 million. One-off costs for recruitment and severance of NOK 3.7 million taken in the quarter. EBITDA margin
P R E S S R E L E A S E
 P R E S S R E L E A S E from ASSA ABLOY AB (publ) 9 August 2002 No. 11/02 INTERIM REPORT FOR THE SECOND QUARTER OF 2002 Sales increased 14% greater focus on organic growth Income before tax increased 26%
P R E S S R E L E A S E from ASSA ABLOY AB (publ) 9 August 2002 No. 11/02 INTERIM REPORT FOR THE SECOND QUARTER OF 2002 Sales increased 14% greater focus on organic growth Income before tax increased 26%
P R E S S R E L E A S E from ASSA ABLOY AB (publ)
 P R E S S R E L E A S E from ASSA ABLOY AB (publ) August 10, 2000 no. 14/00 INTERIM REPORT JANUARY-JUNE 2000 Sales increased by 24% to SEK 6,079 M (4,920) Income before tax increased by 44% to SEK 610
P R E S S R E L E A S E from ASSA ABLOY AB (publ) August 10, 2000 no. 14/00 INTERIM REPORT JANUARY-JUNE 2000 Sales increased by 24% to SEK 6,079 M (4,920) Income before tax increased by 44% to SEK 610
PHOTOCURE ASA RESULTS FOR THIRD QUARTER AND THE FIRST NINE MONTHS November 2018
 PHOTOCURE ASA RESULTS FOR THIRD QUARTER AND THE FIRST NINE MONTHS 2018 8 November 2018 Daniel Schneider, President and CEO Ambaw Bellete, President, Photocure Inc. and Head, US Cancer Commercial Operations
PHOTOCURE ASA RESULTS FOR THIRD QUARTER AND THE FIRST NINE MONTHS 2018 8 November 2018 Daniel Schneider, President and CEO Ambaw Bellete, President, Photocure Inc. and Head, US Cancer Commercial Operations
InDex Pharmaceuticals Holding AB (publ)
 InDex Pharmaceuticals Holding AB (publ) Interim report January-March 2018 Novel formulation for oral administration of cobitolimod PERIOD JANUARY-MARCH 2018 Revenues amounted to SEK 0.1 (0.0) million Operating
InDex Pharmaceuticals Holding AB (publ) Interim report January-March 2018 Novel formulation for oral administration of cobitolimod PERIOD JANUARY-MARCH 2018 Revenues amounted to SEK 0.1 (0.0) million Operating
EBITDA for the period, adjusted for currency effects, was SEK 2.8 (-10.0) million.
 INTERIM REPORT JANUARY MARCH 2015 Net sales were SEK 70.8 (44.5) million. EBITDA for the period, adjusted for currency effects, was SEK 2.8 (-10.0) million. Basic earnings per share amounted to SEK -0.06
INTERIM REPORT JANUARY MARCH 2015 Net sales were SEK 70.8 (44.5) million. EBITDA for the period, adjusted for currency effects, was SEK 2.8 (-10.0) million. Basic earnings per share amounted to SEK -0.06
C o n t i n u e d p r o g r e s s
 Interim report C o n t i n u e d p r o g r e s s Kitron maintained its improvement from the first quarter and returned to the black in the second quarter. The group is on the right course for meeting its
Interim report C o n t i n u e d p r o g r e s s Kitron maintained its improvement from the first quarter and returned to the black in the second quarter. The group is on the right course for meeting its
Highlights. 2 nd quarter and first half 2018 / KEY FIGURES Q2 2018
 Highlights 2 nd quarter and first half 2018 / KEY FIGURES Q2 2018 Revenues of NOK 827 million in 2018, an increase of 42% EBITDA of NOK 65 million in 2018, an increase of 51% Order backlog of NOK 3,178
Highlights 2 nd quarter and first half 2018 / KEY FIGURES Q2 2018 Revenues of NOK 827 million in 2018, an increase of 42% EBITDA of NOK 65 million in 2018, an increase of 51% Order backlog of NOK 3,178
Telio Holding ASA. 4th QUARTER REPORT 2012
 Telio Holding ASA 4th QUARTER REPORT 2012 Telio Holding ASA 4 th Quarter Report 2012 Summary The fourth quarter had a record high customer intake of 15,678 net new customers and organic revenue growth
Telio Holding ASA 4th QUARTER REPORT 2012 Telio Holding ASA 4 th Quarter Report 2012 Summary The fourth quarter had a record high customer intake of 15,678 net new customers and organic revenue growth
FOURTH QUARTER Highlights from fourth quarter 2008 include:
 FOURTH QUARTER 2008 Highlights from fourth quarter 2008 include: Revenues of 1076 MNOK (947 MNOK in fourth quarter 2007). Positive currency impact by 18% Operating profit of 136 MNOK (131 MNOK in fourth
FOURTH QUARTER 2008 Highlights from fourth quarter 2008 include: Revenues of 1076 MNOK (947 MNOK in fourth quarter 2007). Positive currency impact by 18% Operating profit of 136 MNOK (131 MNOK in fourth
Interim report Third quarter of 2012
 Interim report Third quarter of 2012 1 Main features of the third quarter: Oslo Børs strengthens its position in the Nordic market with the acquisition of Burgundy AB High level of activity in the fixed
Interim report Third quarter of 2012 1 Main features of the third quarter: Oslo Børs strengthens its position in the Nordic market with the acquisition of Burgundy AB High level of activity in the fixed
Results and financial position
 Year End Report Results and financial position Year-end Report - Summary: Key Ratios Dignitana Group Net revenues, TSEK 4 069 277 8 902 4 749 Total revenues TSEK 4 189 441 9 122 5 801 Net profit after
Year End Report Results and financial position Year-end Report - Summary: Key Ratios Dignitana Group Net revenues, TSEK 4 069 277 8 902 4 749 Total revenues TSEK 4 189 441 9 122 5 801 Net profit after
Management s Discussion and Analysis of Financial Condition and Results of Operations of Profound Medical Corp. for the Year Ended December 31, 2015
 Management s Discussion and Analysis of Financial Condition and Results of Operations of Profound Medical Corp. for the Year Ended December 31, 2015 The following Management s Discussion and Analysis (
Management s Discussion and Analysis of Financial Condition and Results of Operations of Profound Medical Corp. for the Year Ended December 31, 2015 The following Management s Discussion and Analysis (
Enzon Reports Third Quarter 2006 Results
 November 2, 2006 Enzon Reports Third Quarter 2006 Results Turnaround Makes Visible Progress BRIDGEWATER, N.J., Nov 02, 2006 (BUSINESS WIRE) -- Enzon Pharmaceuticals, Inc. (Nasdaq:ENZN) today announced
November 2, 2006 Enzon Reports Third Quarter 2006 Results Turnaround Makes Visible Progress BRIDGEWATER, N.J., Nov 02, 2006 (BUSINESS WIRE) -- Enzon Pharmaceuticals, Inc. (Nasdaq:ENZN) today announced
AINMT Scandinavia Holdings AS. Quarterly Report January - June
 Quarterly Report January - June 2 0 1 6 Quarterly report SECOND QUARTER SUMMARY - Service revenue of NOK 194,257 thousands; 19% y-o-y growth - EBITDA* of NOK -76,232 thousands - Book equity of NOK 534
Quarterly Report January - June 2 0 1 6 Quarterly report SECOND QUARTER SUMMARY - Service revenue of NOK 194,257 thousands; 19% y-o-y growth - EBITDA* of NOK -76,232 thousands - Book equity of NOK 534
Func Food Group Financial Release / Q2 2017
 Func Food Group Financial Release / Q2 2017 Func Food Group Financial Release / Q2 2017 Func Food Group / Q2 2017 3 FUNC FOOD GROUP IN BRIEF Func Food Group ( FFG ) is a Nordic wellness company, which
Func Food Group Financial Release / Q2 2017 Func Food Group Financial Release / Q2 2017 Func Food Group / Q2 2017 3 FUNC FOOD GROUP IN BRIEF Func Food Group ( FFG ) is a Nordic wellness company, which
INTERIM REPORT JANUARY JUNE 2018 APRIL JUNE 2018 SIGNIFICANT EVENTS. Net sales distribution January-June 2018 (2017) Quarterly net sales
 INTERIM REPORT JANUARY JUNE 2018 Net sales amounted to SEK 184.2 (159.8) million EBITDA was SEK 13.7 (1.2) million Basic earnings per share were SEK -0.06 (-0.18) APRIL JUNE 2018 Net sales amounted to
INTERIM REPORT JANUARY JUNE 2018 Net sales amounted to SEK 184.2 (159.8) million EBITDA was SEK 13.7 (1.2) million Basic earnings per share were SEK -0.06 (-0.18) APRIL JUNE 2018 Net sales amounted to
Clinically superior scalp cooling INTERIM REPORT
 Clinically superior scalp cooling Q3 2018 INTERIM REPORT Contents Results and financial position... 3 CEO comments...5 The company...6 The market...7 Business model...9 The product and product development...
Clinically superior scalp cooling Q3 2018 INTERIM REPORT Contents Results and financial position... 3 CEO comments...5 The company...6 The market...7 Business model...9 The product and product development...
Significant events during the period
 Financial Report - Results and financial position Report - Summary: Key Ratios Dignitana Group Q1 Q1 Full year Net revenues, TSEK 2 807 529 4 900 4 472 4 749 Total revenues TSEK 2 843 601 5 001 5 070 5
Financial Report - Results and financial position Report - Summary: Key Ratios Dignitana Group Q1 Q1 Full year Net revenues, TSEK 2 807 529 4 900 4 472 4 749 Total revenues TSEK 2 843 601 5 001 5 070 5
P R E S S R E L E A S E
 P R E S S R E L E A S E from ASSA ABLOY AB (publ) 6 November No. 22 INTERIM REPORT JANUARY - SEPTEMBER Sales increased by 67% to SEK 16,304 M (9,747) Organic growth for comparable units was 4% Income before
P R E S S R E L E A S E from ASSA ABLOY AB (publ) 6 November No. 22 INTERIM REPORT JANUARY - SEPTEMBER Sales increased by 67% to SEK 16,304 M (9,747) Organic growth for comparable units was 4% Income before
MGC Diagnostics Corporation Reports Fiscal 2017 Third Quarter Results
 MGC Diagnostics Corporation 350 Oak Grove Parkway Saint Paul, MN 55127 Telephone: (651) 484-4874 Facsimile: (651) 484-4826 FOR IMMEDIATE RELEASE MGC Diagnostics Corporation Reports Fiscal 2017 Third Quarter
MGC Diagnostics Corporation 350 Oak Grove Parkway Saint Paul, MN 55127 Telephone: (651) 484-4874 Facsimile: (651) 484-4826 FOR IMMEDIATE RELEASE MGC Diagnostics Corporation Reports Fiscal 2017 Third Quarter
HIGHLIGHTS INTERIM REPORT Q XXL ASA. YTD Growth. Q4 Growth
 INTERIM REPORT Q4 2017 XXL ASA HIGHLIGHTS Total revenues of NOK 2 525 million (NOK 2 151 million), up 17 per cent Like-for-like growth of 7 per cent EBITDA of NOK 332 million (NOK 286 million) Strong cash
INTERIM REPORT Q4 2017 XXL ASA HIGHLIGHTS Total revenues of NOK 2 525 million (NOK 2 151 million), up 17 per cent Like-for-like growth of 7 per cent EBITDA of NOK 332 million (NOK 286 million) Strong cash
Hematology is in our blood
 Hematology is in our blood Boule Diagnostics AB Company presentation, Q4 report 2018 February 7, 2018 Fredrik Dalborg, CEO and Group President Christina Rubenhag, CFO 2019-02-07 BOULE DIAGNOSTICS (1) Copyright
Hematology is in our blood Boule Diagnostics AB Company presentation, Q4 report 2018 February 7, 2018 Fredrik Dalborg, CEO and Group President Christina Rubenhag, CFO 2019-02-07 BOULE DIAGNOSTICS (1) Copyright
THIRD QUARTER Highlights from third quarter 2005 include: Operating profit of 79 MNOK before restructuring charges (83 MNOK last year)
 THIRD QUARTER 2005 Highlights from third quarter 2005 include: Revenues of 701 MNOK (+5% percent relative to third quarter 2004) Operating profit of 79 MNOK before restructuring charges (83 MNOK last year)
THIRD QUARTER 2005 Highlights from third quarter 2005 include: Revenues of 701 MNOK (+5% percent relative to third quarter 2004) Operating profit of 79 MNOK before restructuring charges (83 MNOK last year)
equal to a 19 % (20) operating margin Order intake was SEK 336 m (328), corresponding to an increase of 3 %
 Second quarter Net sales for the second quarter reached SEK 329 m (299), corresponding to an increase of 10 % Operating profit reached SEK 63 m (59) equal to a 19 % (20) operating margin Order intake was
Second quarter Net sales for the second quarter reached SEK 329 m (299), corresponding to an increase of 10 % Operating profit reached SEK 63 m (59) equal to a 19 % (20) operating margin Order intake was
