RE: Medicare Coverage Gap Discount Program Appeals Guidance
|
|
|
- Marvin Richards
- 5 years ago
- Views:
Transcription
1 Cynthia G. Tudor, Ph.D., Director, Medicare Drug Benefit and C & D Data Group Centers for Medicare and Medicaid Services Department of Health and Human Services 7500 Security Boulevard Baltimore, Maryland RE: Medicare Coverage Gap Discount Program Appeals Guidance The Biotechnology Industry Organization (BIO) appreciates this opportunity to comment on the Centers for Medicare & Medicaid Services (CMS) draft guidance on the bases for appeals under the Medicare Coverage Gap Discount Program ( Draft Appeals Guidance ). BIO is the largest trade organization to serve and represent the biotechnology industry in the United States and around the world. BIO represents more than 1,100 biotechnology centers, academic institutions, state biotechnology centers, and related organizations in the United States and in more than 30 other nations. BIO members are involved in the research and development of health care, agricultural, industrial and environmental biotechnology products. BIO represents an industry that is devoted to discovering and ensuring patient access to new and innovative therapies. Many of the therapies developed by biotechnology companies target conditions that primarily affect seniors. BIO has been a strong supporter of the Medicare Part D prescription drug benefit, and believes that the Part D benefit has helped to increase patient access to critical therapies as well as ensure that patients will be able to receive and afford the treatments that best meet their needs. We appreciate CMS s efforts in implementing the Coverage Gap Discount Program.
2 Page 2 of 7 I. Process for Appeals A. Timeframe for Appealing to IRE In explaining the opportunity to seek an additional level of review from the independent review entity (IRE), CMS states that a manufacturer may only initiate this second level of review after a disposition of a dispute submission by the [third party administrator] TPA or when the TPA does not issue a finding within 60 days. 1 This leaves open the possibility that a manufacturer may need to seek an appeal without having the benefit of a disposition from the TPA. We urge CMS to require the TPA to issue a notice to the manufacturer within 60 days of the original dispute in circumstances where the TPA will not be issuing a disposition of a dispute within the required timeframe. This will minimize circumstances in which manufacturers must submit an appeal to the IRE in order to meet the required timeframe for filing such appeals, but the TPA then issues a disposition at the last minute which the manufacturer would not otherwise appeal. Requiring the TPA to notify a manufacturer where the 60 day timeframe will not be met will increase the efficiency of the process and reduce unnecessary consideration of appeals by the IRE. B. Appeals to the Administrator CMS notes that when a manufacturer appeals an IRE decision to the CMS Administrator, the Administrator has the discretion to elect or decline to review the IRE decision. 2 We urge CMS to instead state that the Administrator will make a decision regarding an appeal, and provide a basis for that decision. Not doing so, we believe, is contrary to the requirements in the Model Agreement. 1 Draft Appeals Guidance at 2. 2 Id. at 4.
3 Page 3 of 7 Certainly, the Administrator may determine that the IRE s decision should be upheld, but the purpose of a third round of appeal is to provide manufacturers with a meaningful opportunity to have a dispute heard. If the Administrator is not even required to actually consider the appeal, this in essence removes a level of appeal. Furthermore, the Draft Appeals Guidance also states that if the Administrator does not make a determination regarding review within 30 days, the IRE decision is final and binding. Again, we do not believe this is an appropriate standard. Instead, the Administrator should issue a decision on whether the IRE determination was appropriate. A manufacturer should not be deprived a level of appeal simply because the Administrator was not able to respond within 30 days. II. Bases for Appeal CMS states in the Draft Appeals Guidance that the burden of proof that the submitted data is in error is on the manufacturer and that manufacturers must demonstrate why the information provided with the original dispute demonstrates that a discount payment is in error. 3 Given that manufacturers are provided very limited information on the invoices, we do not believe this standard is appropriate. Certainly, manufacturers should seek appeals only where the manufacturer in good faith believes that the information provided is in error, but, given the limited information provided to manufacturers under the Coverage Gap Discount Program, in many cases manufacturers may not have the information necessary to make such a demonstration. The purpose of an appeal is to allow for another level of review where a dispute has not been satisfactorily resolved; this is set forth in the statute, which does not contemplate that manufacturers must bear the burden of proof. 4 3 Id. 4 Social Security Act 1860D-14A(c)(1)(A)(vii).
4 Page 4 of 7 III. Examples of Bases for Appeals CMS provides several examples of reasons that manufacturers may seek an appeal, along with guidance on the types of evidence CMS expects manufacturers will submit when pursuing such appeals. We urge CMS first to make clear that the four reasons listed in the Draft Appeals Guidance are not the only reasons that a manufacturer may appeal the accuracy of an invoice. Such a clarification would be consistent with the multiple data field options the TPA has provided as reason codes for the dispute, which contemplate that an invoice may be disputed for a range of reasons; many of these reasons may also be legitimate reasons for appeal to an IRE. We also urge CMS to clarify that a manufacturer may submit more than one reason for an initial dispute. While not indicated in the Draft Appeals Guidance, we understand from the webinars and associated materials provided by the TPA that a manufacturer may only be permitted to indicate a single reason for any specific dispute. We urge CMS to clarify that where there are multiple reasons for a dispute, a manufacturer may appropriately indicate all of the reasons for the dispute in the appropriate field. This is particularly important if CMS maintains its proposed policy that all information relevant to the appeal must be submitted at the time of the appeal and that no additional information may be submitted in subsequent rounds of the appeal. With respect to the particular examples of reasons a manufacturer may dispute an invoice that CMS describes in the Draft Appeals Guidance, we provide the following comments: A. Aberrant Quantity CMS states that [m]anufacturers who consider product dosages, whether more or less than three times the maximum FDA dosages, to be aberrant should be prepared to demonstrate to the satisfaction of the IRE that the dosage represents a
5 Page 5 of 7 severe threat to the health of beneficiaries. 5 We note that the purpose of the appeals process is to ensure that Coverage Gap Discount Program payments are accurate. We believe that the standard that a manufacturer only engage in an appeal where it has a good faith reason to believe that an invoice is in error should be sufficient for the IRE to consider an appeal regarding a disputed invoice. It is quite possible that a quantity could be incorrect without the quantity representing a severe threat to the health of beneficiaries. This standard is not appropriate for a payment dispute. Whether a manufacturer was properly billed for a discount payment does not hinge on whether the quantity, even if accurately reflected on the invoices, would present a severe threat to beneficiary health. We urge CMS to reconsider this standard and instead adopt standards that more appropriately reflect the nature of the these disputes, which relate to payments that may result from inaccurate invoices. B. Part B Drugs Ineligible for Discount As CMS notes in the Draft Appeals Guidance, many prescription drug products that are covered under Medicare Part B may also be covered under Medicare Part D depending upon the indication or provider setting. 6 CMS proposes in the Draft Appeals Guidance that a manufacturer may appeal to an IRE in these types of situations where the manufacturer demonstrates that the applicable drug would not be covered under Part D because the Service Provider indicated on the detailed Manufacturer Data Report does not represent a pharmacy. 7 We urge CMS not to use such a standard as a threshold for an appeal to an IRE. Manufacturers will not always have enough information to determine 5 Draft Appeals Guidance at 3 (emphasis added). 6 Id. 7 Id.
6 Page 6 of 7 whether a drug is properly dispensed under Part B or Part D based upon the service provider. Drugs typically are covered under Part B versus Part D based on the manner dispensed and prescribed to a particular patient. While in many cases a therapy dispensed in a retail pharmacy is appropriately paid for under Part D, this is not always the case. For example, in some cases a retail pharmacy may dispense a therapy that usually is covered under Part B as part of durable medical equipment but may be covered under Part D in other circumstances. The information provided to manufacturers as part of the Coverage Gap Discount Program would not allow a manufacturer to determine whether a drug was dispensed to a patient under Part B rather than Part D. While we expect that the majority of such circumstances would be resolved as the TPA reviews a disputed invoice, a manufacturer should be able to appeal a TPA determination if it is not satisfied that the TPA has provided sufficient response to support its determination that Part D coverage was appropriate. Indeed, in some cases, as contemplated by the Draft Appeals Guidance, a manufacturer will not even have the benefit of a TPA determination before having to file an appeal in order to meet the required timeframes. Where a manufacturer identifies a disproportionate number of Part D claims for a drug that most commonly is covered under Part B, the only way for a manufacturer to verify the accuracy of the coverage gap discount invoices is to dispute such an invoice and, where appropriate, to appeal an IRE determination, as is provided for by the statute. IV. Implementation of the Draft Appeals Guidance BIO encourages CMS to delay finalizing the appeals guidance until at least sixty days after the first quarter of invoices have been issued. This will allow manufacturers to view the first batch of invoices and better understand the types of outliers that may be appearing. This would better inform the kinds of issues that may arise in the dispute and appeals processes. Alternatively, we urge CMS to provide another opportunity for comment on the appeals process after the first year of the program. This will allow CMS to incorporate feedback from stakeholders
7 Page 7 of 7 based on the program s initial experience and to update the appeals process in a manner that will be more efficient for all parties. We also ask that CMS consider an extended timeframe for disputing invoices for the first few quarters. Given that this is a new program, this would provide manufacturers with the time necessary to adequately review and understand the data being provided with the initial invoices, while at the same time issuing the first few rounds of payments. An extended timeframe for the first few quarters is likely to reduce the number of disputes, as manufacturers will have the opportunity to fully review and understand the information provided. Conclusion BIO appreciates the opportunity to comment on the draft guidance on the Draft Appeals Guidance. We look forward to continuing to work with CMS to address these critical issues in the future. Please feel free to contact Laurel Todd at (202) if you have any questions or if we can be of further assistance. Thank you for your attention to this very important matter. Respectfully submitted, /s/ Laurel Todd Managing Director Reimbursement and Health Policy
Re: Medicare Prescription Drug Benefit Manual Draft Chapter 6
 September 26, 2006 BY ELECTRONIC DELIVERY Cynthia Tudor, Ph.D. Director, Medicare Drug Benefit Group Centers for Medicare & Medicaid Services Mail Stop C4-13-01 7500 Security Boulevard Baltimore, MD 21244
September 26, 2006 BY ELECTRONIC DELIVERY Cynthia Tudor, Ph.D. Director, Medicare Drug Benefit Group Centers for Medicare & Medicaid Services Mail Stop C4-13-01 7500 Security Boulevard Baltimore, MD 21244
Re: Medicare Prescription Drug Benefit Manual Draft Chapter 5
 September 18, 2006 BY ELECTRONIC DELIVERY Cynthia Tudor, Ph.D. Director, Medicare Drug Benefit Group Centers for Medicare and Medicaid Services Department of Health and Human Services Mail Stop C4-13-01
September 18, 2006 BY ELECTRONIC DELIVERY Cynthia Tudor, Ph.D. Director, Medicare Drug Benefit Group Centers for Medicare and Medicaid Services Department of Health and Human Services Mail Stop C4-13-01
TX Health and Human Services Commission Proposed Rule: 340B Program Reimbursement
 January 31, 2014 VIA ELECTRONIC SUBMISSION Vendor Drug Program Medicaid/CHIP Division 4900 N. Lamar Austin, Texas 78751 RE: TX Health and Human Services Commission Proposed Rule: 340B Program Reimbursement
January 31, 2014 VIA ELECTRONIC SUBMISSION Vendor Drug Program Medicaid/CHIP Division 4900 N. Lamar Austin, Texas 78751 RE: TX Health and Human Services Commission Proposed Rule: 340B Program Reimbursement
June 30, 2006 BY ELECTRONIC DELIVERY
 June 30, 2006 BY ELECTRONIC DELIVERY Mark McClellan, M.D., Ph.D., Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Room 445-G Hubert H. Humphrey Building
June 30, 2006 BY ELECTRONIC DELIVERY Mark McClellan, M.D., Ph.D., Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Room 445-G Hubert H. Humphrey Building
A. As Currently Implemented, the Recovery Purchasing Program Is Not Truly Voluntary for FSS Contractors Under Schedule 65, Part I, Section B.
 April 2, 2007 Ms. Laurieann Duarte General Services Administration Regulatory Secretariat (VIR) 1800 F Street, NW Room 4035 Washington, D.C. 20405 Dear Ms. Duarte: Re: Amendment 2007-01, GSAR Case 2006-G522;
April 2, 2007 Ms. Laurieann Duarte General Services Administration Regulatory Secretariat (VIR) 1800 F Street, NW Room 4035 Washington, D.C. 20405 Dear Ms. Duarte: Re: Amendment 2007-01, GSAR Case 2006-G522;
Re: CMS-1502-P (Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule for Calendar Year 2006)
 BY ELECTRONIC DELIVERY Mark McClellan, Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Room 445-G Hubert H. Humphrey Building 200 Independence Avenue, S.W.
BY ELECTRONIC DELIVERY Mark McClellan, Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Room 445-G Hubert H. Humphrey Building 200 Independence Avenue, S.W.
Solicitation of Public Comments on the Protecting Access to Medicare Act (PAMA)
 ASSOCIATION FOR MOLECULAR PATHOLOGY Education. Innovation & Improved Patient Care. Advocacy. 9650 Rockville Pike, Suite 205, Bethesda, Maryland 20814 Tel: 301-634-7939 Fax: 301-634-7995 amp@amp.org www.amp.org
ASSOCIATION FOR MOLECULAR PATHOLOGY Education. Innovation & Improved Patient Care. Advocacy. 9650 Rockville Pike, Suite 205, Bethesda, Maryland 20814 Tel: 301-634-7939 Fax: 301-634-7995 amp@amp.org www.amp.org
MEDICARE PLAN PAYMENT GROUP
 DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, Maryland 21244-1850 MEDICARE PLAN PAYMENT GROUP Date: June 23, 2017 To: From: All Part
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, Maryland 21244-1850 MEDICARE PLAN PAYMENT GROUP Date: June 23, 2017 To: From: All Part
Continuation of the Prescription Drug Event (PDE) Reports and PDE Analysis Reporting Initiatives for the 2014 Benefit Year
 DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Center for Medicare 7500 Security Boulevard Baltimore, Maryland 21244-1850 Center for Medicare Medicare Plan Payment Group
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Center for Medicare 7500 Security Boulevard Baltimore, Maryland 21244-1850 Center for Medicare Medicare Plan Payment Group
March 3, VIA Electronic Filing:
 March 3, 2017 VIA Electronic Filing: AdvanceNotice2018@cms.hhs.gov Cynthia G. Tudor, PhD Acting Administrator Centers for Medicare & Medicaid Services 7500 Security Blvd. Baltimore, Maryland 21244 Dear
March 3, 2017 VIA Electronic Filing: AdvanceNotice2018@cms.hhs.gov Cynthia G. Tudor, PhD Acting Administrator Centers for Medicare & Medicaid Services 7500 Security Blvd. Baltimore, Maryland 21244 Dear
VERMONT SUPPLEMENTAL DRUG-REBATE AGREEMENT
 VERMONT SUPPLEMENTAL DRUG-REBATE AGREEMENT 1.1 This Supplemental Drug-Rebate Agreement ("Agreement") is made and entered into this day of, by and between the State of Vermont, Department of Vermont Health
VERMONT SUPPLEMENTAL DRUG-REBATE AGREEMENT 1.1 This Supplemental Drug-Rebate Agreement ("Agreement") is made and entered into this day of, by and between the State of Vermont, Department of Vermont Health
MEDICARE PLAN PAYMENT GROUP
 DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, Maryland 21244-1850 MEDICARE PLAN PAYMENT GROUP Date: May 30, 2018 To: From: All Part D
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, Maryland 21244-1850 MEDICARE PLAN PAYMENT GROUP Date: May 30, 2018 To: From: All Part D
PURPOSE OF THE POLICY STATEMENT OF THE POLICY PROCEDURES
 PURPOSE OF THE POLICY The purpose of this policy is to describe Health Alliance s process for transitions and ensure that continued drug coverage is provided to new and current Part D members. The transition
PURPOSE OF THE POLICY The purpose of this policy is to describe Health Alliance s process for transitions and ensure that continued drug coverage is provided to new and current Part D members. The transition
Values Accountability Integrity Service Excellence Innovation Collaboration
 n04231 Medicare Part D Transition and Emergency Fill Policy Values Accountability Integrity Service Excellence Innovation Collaboration Abstract Purpose: The Medicare Part D Transition and Emergency Fill
n04231 Medicare Part D Transition and Emergency Fill Policy Values Accountability Integrity Service Excellence Innovation Collaboration Abstract Purpose: The Medicare Part D Transition and Emergency Fill
This section has been included to provide an overview of NLPDP Provider Audit practices, policies, and procedures.
 12. AUDIT OF CLAIMS 12.1 OVERVIEW This section has been included to provide an overview of NLPDP Provider Audit practices, policies, and procedures. Providers are entitled to payment for eligible claims.
12. AUDIT OF CLAIMS 12.1 OVERVIEW This section has been included to provide an overview of NLPDP Provider Audit practices, policies, and procedures. Providers are entitled to payment for eligible claims.
WYOMING MEDICAID SUPPLEMENTAL DRUG REBATE AGREEMENT
 SSDC WYOMING MEDICAID SUPPLEMENTAL DRUG REBATE AGREEMENT 1. PARTIES/PERIOD This Agreement is made and entered into this 1 st day of January, 2012, by and between the State of Wyoming (State), represented
SSDC WYOMING MEDICAID SUPPLEMENTAL DRUG REBATE AGREEMENT 1. PARTIES/PERIOD This Agreement is made and entered into this 1 st day of January, 2012, by and between the State of Wyoming (State), represented
December 20, Howard A. Zucker, M.D., J.D. Commissioner Department of Health Corning Tower Empire State Plaza Albany, NY 12237
 December 20, 2017 Howard A. Zucker, M.D., J.D. Commissioner Department of Health Corning Tower Empire State Plaza Albany, NY 12237 Re: Optimizing Medicaid Drug Rebates Report 2017-F-9 Dear Dr. Zucker:
December 20, 2017 Howard A. Zucker, M.D., J.D. Commissioner Department of Health Corning Tower Empire State Plaza Albany, NY 12237 Re: Optimizing Medicaid Drug Rebates Report 2017-F-9 Dear Dr. Zucker:
Implement a definition of negotiated price to include all pharmacy price concessions.
 NCPA Analysis of Medicare Part D Pharmacy DIR Fee Reform Policy Proposal and Other Policies Impacting Community Pharmacies in the CMS Proposed Rule, Modernizing Part D and Medicare Advantage to Lower Drug
NCPA Analysis of Medicare Part D Pharmacy DIR Fee Reform Policy Proposal and Other Policies Impacting Community Pharmacies in the CMS Proposed Rule, Modernizing Part D and Medicare Advantage to Lower Drug
Medicare Part D Transition Policy CY 2018 HCSC Medicare Part D
 Contract: H0107, H0927, H1666, H3251, H3822, H3979, H8133, H8634, H8554, S5715 Policy Name: Medicare Formulary Transition Purpose: This procedure describes the standard process Health Care Service Corporation
Contract: H0107, H0927, H1666, H3251, H3822, H3979, H8133, H8634, H8554, S5715 Policy Name: Medicare Formulary Transition Purpose: This procedure describes the standard process Health Care Service Corporation
T MaxorPlus Pharmacy Provider Manual
 T MaxorPlus Pharmacy Provider Manual March 2017 320 SOUTH POLK, SUITE 200 AMARILLO, TEXAS 79101 PHONE: (800) 658-6146 FAX: (806) 324-5486 WWW.MAXORPLUS.COM 1 MaxorPlus Pharmacy Provider Manual Table of
T MaxorPlus Pharmacy Provider Manual March 2017 320 SOUTH POLK, SUITE 200 AMARILLO, TEXAS 79101 PHONE: (800) 658-6146 FAX: (806) 324-5486 WWW.MAXORPLUS.COM 1 MaxorPlus Pharmacy Provider Manual Table of
Subject: Plan Finder Observations During Fall Open Enrollment: October 15 December 7, 2013 Date: May 16, 2014
 520 Eighth Avenue, North Wing, 3rd Floor New York, NY 10018 212.869.3850/Fax: 212.869.3532 MEMORANDUM To: From: Arrah Tabe-Bedward, Director, Medicare Enrollment and Appeals Group Amy Larrick, Acting Director,
520 Eighth Avenue, North Wing, 3rd Floor New York, NY 10018 212.869.3850/Fax: 212.869.3532 MEMORANDUM To: From: Arrah Tabe-Bedward, Director, Medicare Enrollment and Appeals Group Amy Larrick, Acting Director,
Via Electronic Submission (www.regulations.gov) January 16, 2018
 Via Electronic Submission (www.regulations.gov) January 16, 2018 Ms. Seema Verma Administrator Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services ATTN: CMS-4182-P 7500
Via Electronic Submission (www.regulations.gov) January 16, 2018 Ms. Seema Verma Administrator Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services ATTN: CMS-4182-P 7500
2018 Medicare Part D Transition Policy
 Regulation/ Requirements Purpose Scope Policy 2018 Medicare Part D Transition Policy 42 CFR 423.120(b)(3) 42 CFR 423.154(a)(1)(i) 42 CFR 423.578(b) Medicare Prescription Drug Benefit Manual, Chapter 6,
Regulation/ Requirements Purpose Scope Policy 2018 Medicare Part D Transition Policy 42 CFR 423.120(b)(3) 42 CFR 423.154(a)(1)(i) 42 CFR 423.578(b) Medicare Prescription Drug Benefit Manual, Chapter 6,
2019 Transition Policy
 2019 Number: 5.8 Prescription Drug Replaces: 5.8 v.2018 Cross 5.1.2 Transition Fill Monitoring Procedure References: Purpose: To provide guidance on the transition process for new or current Plan members
2019 Number: 5.8 Prescription Drug Replaces: 5.8 v.2018 Cross 5.1.2 Transition Fill Monitoring Procedure References: Purpose: To provide guidance on the transition process for new or current Plan members
Summary of 2017 Medicare Part D Final Call Letter
 Summary of 2017 Medicare Part D Final Call Letter On April 4, 2016, the Centers for Medicare & Medicaid Services (CMS) issued the 2017 Medicare Advantage Capitation Rates and Medicare Advantage and Part
Summary of 2017 Medicare Part D Final Call Letter On April 4, 2016, the Centers for Medicare & Medicaid Services (CMS) issued the 2017 Medicare Advantage Capitation Rates and Medicare Advantage and Part
SUPPLEMENTAL REBATE AGREEMENT Company Name
 Department Log # SUPPLEMENTAL REBATE AGREEMENT Company Name This Supplemental Rebate Agreement ( Agreement ) is dated as of this 1 st day of January, by and between the State of Utah Department of Health,
Department Log # SUPPLEMENTAL REBATE AGREEMENT Company Name This Supplemental Rebate Agreement ( Agreement ) is dated as of this 1 st day of January, by and between the State of Utah Department of Health,
Compensation and Reimbursement
 492 Pharmacy Management: Compensation and Reimbursement Positions Compensation and Reimbursement Revenue Cycle Compliance and Management (1710) To encourage pharmacists to serve as leaders in the development
492 Pharmacy Management: Compensation and Reimbursement Positions Compensation and Reimbursement Revenue Cycle Compliance and Management (1710) To encourage pharmacists to serve as leaders in the development
COALITION FOR WHOLE HEALTH
 COALITION FOR WHOLE HEALTH June 9, 2015 Andy Slavitt, Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services 7500 Security Boulevard Baltimore, Maryland 21244
COALITION FOR WHOLE HEALTH June 9, 2015 Andy Slavitt, Acting Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services 7500 Security Boulevard Baltimore, Maryland 21244
Sent via electronic transmission to:
 March 3, 2017 Patrick Conway, MD Acting Administrator Centers for Medicare and Medicaid Services US Department of Health and Human Services 200 Independence Avenue, SW Washington, DC 20201 Sent via electronic
March 3, 2017 Patrick Conway, MD Acting Administrator Centers for Medicare and Medicaid Services US Department of Health and Human Services 200 Independence Avenue, SW Washington, DC 20201 Sent via electronic
Employer Group Waiver Plan (EGWP) FAQs
 EGWP: An opportunity for Alaska to maintain existing pharmacy benefits for Medicare-eligible retirees and achieve cost savings for years to come. An Employer Group Waiver Plan, known as an EGWP or Egg
EGWP: An opportunity for Alaska to maintain existing pharmacy benefits for Medicare-eligible retirees and achieve cost savings for years to come. An Employer Group Waiver Plan, known as an EGWP or Egg
CDHP Special Administration
 CDHP Special Administration Your prescription coverage under the Consumer Driven Health Plan (CDHP) is subject to special administration from the PPO plans and this page will explain those differences:
CDHP Special Administration Your prescription coverage under the Consumer Driven Health Plan (CDHP) is subject to special administration from the PPO plans and this page will explain those differences:
March 4, 2016 BY ELECTRONIC DELIVERY
 BY ELECTRONIC DELIVERY Sean Cavanaugh Deputy Administrator Director, Center for Medicare Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, MD 21244 cc: Jennifer Wuggazer Lazio,
BY ELECTRONIC DELIVERY Sean Cavanaugh Deputy Administrator Director, Center for Medicare Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, MD 21244 cc: Jennifer Wuggazer Lazio,
Medicaid Program; Announcement of Medicaid Drug Rebate Program National Rebate. AGENCY: Centers for Medicare & Medicaid Services (CMS), HHS.
 This document is scheduled to be published in the Federal Register on 03/23/2018 and available online at https://federalregister.gov/d/2018-05947, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
This document is scheduled to be published in the Federal Register on 03/23/2018 and available online at https://federalregister.gov/d/2018-05947, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
PEP-Portland Clinical Practices Policy Number: CP Policy Owner: Health Plan Operations Manager New Revised Reviewed
 Subject: Transition Process for Medicare Part D Approval Group: Pharmacy Management Group Signed By: Ellen Garcia, Executive Director Policy Number: CP5500.120 Policy Owner: Health Plan Operations Manager
Subject: Transition Process for Medicare Part D Approval Group: Pharmacy Management Group Signed By: Ellen Garcia, Executive Director Policy Number: CP5500.120 Policy Owner: Health Plan Operations Manager
SUBMISSION OF PUBLIC COMMENTS:
 Request for Information: Performance Indicators for Medicaid and Children s Health Insurance Program (CHIP) Business Functions: Solicitation of Public Input This solicitation seeks public input to aid
Request for Information: Performance Indicators for Medicaid and Children s Health Insurance Program (CHIP) Business Functions: Solicitation of Public Input This solicitation seeks public input to aid
I. PURPOSE. A. The primary objectives of Molina Healthcare s Transition Policy and Procedure are:
 I. PURPOSE The purpose of the Policy and Procedure is to ensure necessary continuity of treatment and to provide adequate time and transition process to introduce the enrollee and their prescribing physician
I. PURPOSE The purpose of the Policy and Procedure is to ensure necessary continuity of treatment and to provide adequate time and transition process to introduce the enrollee and their prescribing physician
All Medicare Advantage Plans, Prescription Drug Plans, Section 1876 Cost Plans, Medicare-Medicaid Plans, and PACE Organizations
 DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard, Mail Stop C1-22-06 Baltimore, Maryland 21244-1850 MEDICARE PARTS C AND D OVERSIGHT AND ENFORCEMENT
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard, Mail Stop C1-22-06 Baltimore, Maryland 21244-1850 MEDICARE PARTS C AND D OVERSIGHT AND ENFORCEMENT
MEDICARE PART D POLICY FORMULARY: TRANSITION PROCESS Policy Number: 6-C
 MEDICARE PART D POLICY FORMULARY: TRANSITION PROCESS Policy Number: 6-C Coverage Statement This Policy is applicable to: Medco PDP, Beneficiaries, Enhanced PDPs, Client PDPs and Client MA-PDs, to the extent
MEDICARE PART D POLICY FORMULARY: TRANSITION PROCESS Policy Number: 6-C Coverage Statement This Policy is applicable to: Medco PDP, Beneficiaries, Enhanced PDPs, Client PDPs and Client MA-PDs, to the extent
Part II: Medicare Part C and Part D
 Part II: Medicare Part C and Part D Part II: Part C and Part D Part C (Medicare Advantage)... 1 Enhanced Payments to Plans for Certain Beneficiary Types... 1 Special Needs Plans: Enrollment of Medicare
Part II: Medicare Part C and Part D Part II: Part C and Part D Part C (Medicare Advantage)... 1 Enhanced Payments to Plans for Certain Beneficiary Types... 1 Special Needs Plans: Enrollment of Medicare
March 1, Dear Mr. Kouzoukas:
 March 1, 2019 Mr. Demetrios L. Kouzoukas Principal Deputy Administrator and Director Center for Medicare Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, MD 21244 Re: Advance
March 1, 2019 Mr. Demetrios L. Kouzoukas Principal Deputy Administrator and Director Center for Medicare Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, MD 21244 Re: Advance
REQUEST FOR MEDICARE PRESCRIPTION DRUG COVERAGE DETERMINATION This form may be sent to us by mail or fax:
 REQUEST FOR MEDICARE PRESCRIPTION DRUG COVERAGE DETERMINATION This form may be sent to us by mail or fax: Address: Fax Number: Aetna Better Health of Virginia (HMO SNP) 1-877-270-0148 Part D Coverage Determination
REQUEST FOR MEDICARE PRESCRIPTION DRUG COVERAGE DETERMINATION This form may be sent to us by mail or fax: Address: Fax Number: Aetna Better Health of Virginia (HMO SNP) 1-877-270-0148 Part D Coverage Determination
Appeals and Grievances: What to Do if You Have Complaints About Your Part D Prescription Drug Benefits
 Appeals and Grievances: What to Do if You Have Complaints About Your Part D Prescription Drug Benefits WHAT TO DO IF YOU HAVE COMPLAINTS We encourage you to let us know right away if you have questions,
Appeals and Grievances: What to Do if You Have Complaints About Your Part D Prescription Drug Benefits WHAT TO DO IF YOU HAVE COMPLAINTS We encourage you to let us know right away if you have questions,
SPECIALTY PHARMACY MANDATORY MEASURES
 SPECIALTY PHARMACY MANDATORY S Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis. # DESCRIPTION NUMERATOR DENOMINATOR DTM
SPECIALTY PHARMACY MANDATORY S Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis. # DESCRIPTION NUMERATOR DENOMINATOR DTM
Coverage Gap Discount Program (CGDP) Introduction For Manufacturers October 28, 2010
 Coverage Gap Discount Program (CGDP) Introduction For Manufacturers October 28, 2010 Agenda Introduction and Welcome Objectives Quarterly Payment Information Flow TPA Welcome Letter Draft Report Formats
Coverage Gap Discount Program (CGDP) Introduction For Manufacturers October 28, 2010 Agenda Introduction and Welcome Objectives Quarterly Payment Information Flow TPA Welcome Letter Draft Report Formats
Martin s Point Generations Advantage Policy and Procedure Form
 Martin s Point Generations Advantage Policy and Procedure Form Policy #: PartD.923 Effective Date: 4/16/10 Policy Title: Part D Transition Policy Section of Manual: Medicare Prescription Drug Benefit Manual
Martin s Point Generations Advantage Policy and Procedure Form Policy #: PartD.923 Effective Date: 4/16/10 Policy Title: Part D Transition Policy Section of Manual: Medicare Prescription Drug Benefit Manual
340B Program Contract Pharmacy Self-Audit Tool: Diversion
 Page 1 Purpose: The purpose of the Contract Pharmacy Self-Audit Tools is to improve contract pharmacies compliance with the 340B Program requirements. Covered entities remain responsible for the 340B drugs
Page 1 Purpose: The purpose of the Contract Pharmacy Self-Audit Tools is to improve contract pharmacies compliance with the 340B Program requirements. Covered entities remain responsible for the 340B drugs
MEDICARE PART D PRESCRIPTION DRUG BENEFIT
 MEDICARE PART D PRESCRIPTION DRUG BENEFIT On January 21, 2005, the Centers for Medicare & Medicaid Services ( CMS ) issued the final regulations implementing the Medicare prescription drug benefit as well
MEDICARE PART D PRESCRIPTION DRUG BENEFIT On January 21, 2005, the Centers for Medicare & Medicaid Services ( CMS ) issued the final regulations implementing the Medicare prescription drug benefit as well
ATTN: Comments on 340B Drug Pricing Program Omnibus Guidance
 October 27, 2015 Krista Pedley Director, Office of Pharmacy Affairs Health Resources and Services Administration 5600 Fishers Lane Rockville, MD 20857 ATTN: Comments on 340B Drug Pricing Program Omnibus
October 27, 2015 Krista Pedley Director, Office of Pharmacy Affairs Health Resources and Services Administration 5600 Fishers Lane Rockville, MD 20857 ATTN: Comments on 340B Drug Pricing Program Omnibus
Medi-Pak Advantage: Terms and Conditions of Provider Participation
 Medi-Pak Advantage: Terms and Conditions of Provider Participation Medi-Pak Advantage is a Medicare Advantage Private Fee-For-Service plan offered by Arkansas Blue Cross and Blue Shield. Medi-Pak Advantage
Medi-Pak Advantage: Terms and Conditions of Provider Participation Medi-Pak Advantage is a Medicare Advantage Private Fee-For-Service plan offered by Arkansas Blue Cross and Blue Shield. Medi-Pak Advantage
Prime Perspective. From the auditor s desk. Quarterly Pharmacy Newsletter from Prime Therapeutics LLC INSIDE. March 2019: Issue 75
 Prime Perspective Quarterly Pharmacy Newsletter from Prime Therapeutics LLC March 2019: Issue 75 From the auditor s desk INSIDE From the auditor s desk... 1 2 Medicare news/medicaid news..2 Florida news...3
Prime Perspective Quarterly Pharmacy Newsletter from Prime Therapeutics LLC March 2019: Issue 75 From the auditor s desk INSIDE From the auditor s desk... 1 2 Medicare news/medicaid news..2 Florida news...3
Florida Medicaid. Prescribed Drugs Services Coverage Policy. Agency for Health Care Administration. Draft Rule
 Florida Medicaid Prescribed Drugs Services Coverage Policy Agency for Health Care Administration Draft Rule Table of Contents Introduction... 1 1.1 Description... 1 1.2 Legal Authority... 1 1.3 Definitions...
Florida Medicaid Prescribed Drugs Services Coverage Policy Agency for Health Care Administration Draft Rule Table of Contents Introduction... 1 1.1 Description... 1 1.2 Legal Authority... 1 1.3 Definitions...
Coverage Determinations, Appeals and Grievances
 Coverage Determinations, Appeals and Grievances Filing a grievance (making a complaint) about your prescription coverage Asking for a coverage determination (coverage decision) 60-day formulary change
Coverage Determinations, Appeals and Grievances Filing a grievance (making a complaint) about your prescription coverage Asking for a coverage determination (coverage decision) 60-day formulary change
BY ELECTRONIC MAIL TO
 BY ELECTRONIC MAIL TO NONPROFITIPREGS@CIRM.CA.GOV Mr. C. Scott Tocher Interim Counsel California Institute for Regenerative Medicine 250 King Street San Francisco, CA 94107 Comments to Proposed Changes
BY ELECTRONIC MAIL TO NONPROFITIPREGS@CIRM.CA.GOV Mr. C. Scott Tocher Interim Counsel California Institute for Regenerative Medicine 250 King Street San Francisco, CA 94107 Comments to Proposed Changes
Medicare Transition POLICY AND PROCEDURES
 Medicare Transition POLICY AND PROCEDURES POLICY The Plan will maintain an appropriate transition process, consistent with 42 CFR 423.120(b)(3), Chapter 6 of the Medicare Prescription Drug Benefit Manual
Medicare Transition POLICY AND PROCEDURES POLICY The Plan will maintain an appropriate transition process, consistent with 42 CFR 423.120(b)(3), Chapter 6 of the Medicare Prescription Drug Benefit Manual
SPECIALTY PHARMACY ACCREDITATION V3.0 MANDATORY MEASURES
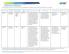 MANDATORY S Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to on an annual basis. # DESCRIPTION NUMERATOR DENOMINATOR DATA SOURCE DM2012-13 Drug-Drug
MANDATORY S Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to on an annual basis. # DESCRIPTION NUMERATOR DENOMINATOR DATA SOURCE DM2012-13 Drug-Drug
SHARP HEALTH PLAN MEDICARE ADVANTAGE POLICY AND PROCEDURE Product Line (check all that apply):
 SHARP HEALTH PLAN MEDICARE ADVANTAGE POLICY AND PROCEDURE Product Line (check all that apply): Title: SHP Pharmacy Management Policy and Procedure for Part D Coverage Determination All Group HMO Individual
SHARP HEALTH PLAN MEDICARE ADVANTAGE POLICY AND PROCEDURE Product Line (check all that apply): Title: SHP Pharmacy Management Policy and Procedure for Part D Coverage Determination All Group HMO Individual
PLEASE CHECK ALL BOXES THAT APPLY AND COMPLETE THE APPROPRIATE SECTION(S) OF THE FORM. Patient name: Date of birth: Sex: M F
 TM RENFLEXIS for injection (inf liximab-abda)100 mg The Merck Access Program ENROLLMENT FORM Before prescribing RENFLEXIS, please read the accompanying Prescribing Information, including the Boxed Warning
TM RENFLEXIS for injection (inf liximab-abda)100 mg The Merck Access Program ENROLLMENT FORM Before prescribing RENFLEXIS, please read the accompanying Prescribing Information, including the Boxed Warning
340B Compliance, Audits & Opportunities
 340B Compliance, Audits & Opportunities NW Ohio HFMA February 15, 2018 David Layne, CPA Manager HRSA Audits Bizzell Group-Silver Spring, Maryland Prior Hospital experience Many are pharmacists Experienced
340B Compliance, Audits & Opportunities NW Ohio HFMA February 15, 2018 David Layne, CPA Manager HRSA Audits Bizzell Group-Silver Spring, Maryland Prior Hospital experience Many are pharmacists Experienced
Product Reimbursement Services and Patient Assistance Programs KATHY CHAURETTE ALESSANDRO MARTUSCELLI
 Product Reimbursement Services and Patient Assistance Programs KATHY CHAURETTE ALESSANDRO MARTUSCELLI Overview of Legal Framework OIG Guidance Pharmaceutical manufacturers may provide certain support services
Product Reimbursement Services and Patient Assistance Programs KATHY CHAURETTE ALESSANDRO MARTUSCELLI Overview of Legal Framework OIG Guidance Pharmaceutical manufacturers may provide certain support services
North Carolina Medicaid Reform Status Briefing
 North Carolina Medicaid Reform Status Briefing Overview Medicaid reform was signed into law by Gov. McCrory in September 2015, after extensive engagement with the General Assembly, providers, beneficiaries
North Carolina Medicaid Reform Status Briefing Overview Medicaid reform was signed into law by Gov. McCrory in September 2015, after extensive engagement with the General Assembly, providers, beneficiaries
Re: CMS 2238 FC (Final Rule: Medicaid Program; Prescription Drugs)
 January 2, 2008 Reference No.: FASC08001 Kerry Weems Acting Administrator, Centers for Medicare and Medicaid Services Department of Health and Human Services Room 445-G Hubert H. Humphrey Building 200
January 2, 2008 Reference No.: FASC08001 Kerry Weems Acting Administrator, Centers for Medicare and Medicaid Services Department of Health and Human Services Room 445-G Hubert H. Humphrey Building 200
Principles for Ensuring Fair and Appropriate Practices for Individual Market Policy Rescissions and Pre-existing Conditions Clauses
 Principles for Ensuring Fair and Appropriate Practices for Individual Market Policy Rescissions and Pre-existing Conditions Clauses The Board of Directors of America s Health Insurance Plans (AHIP) and
Principles for Ensuring Fair and Appropriate Practices for Individual Market Policy Rescissions and Pre-existing Conditions Clauses The Board of Directors of America s Health Insurance Plans (AHIP) and
1 SB By Senators Beasley, Smitherman, Irons, Bussman and Ross. 4 RFD: Health. 5 First Read: 12-APR-11. Page 0
 1 SB390 2 124198-2 3 By Senators Beasley, Smitherman, Irons, Bussman and Ross 4 RFD: Health 5 First Read: 12-APR-11 Page 0 1 124198-2:n:03/21/2011:MCS/ll LRS2010-4156R1 2 3 4 5 6 7 8 SYNOPSIS: Existing
1 SB390 2 124198-2 3 By Senators Beasley, Smitherman, Irons, Bussman and Ross 4 RFD: Health 5 First Read: 12-APR-11 Page 0 1 124198-2:n:03/21/2011:MCS/ll LRS2010-4156R1 2 3 4 5 6 7 8 SYNOPSIS: Existing
SHO # ACA # 22
 DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard, Mail Stop S2-26-12 Baltimore, Maryland 21244-1850 SHO # 12-003 ACA # 22 December 28, 2012 RE: Conversion
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard, Mail Stop S2-26-12 Baltimore, Maryland 21244-1850 SHO # 12-003 ACA # 22 December 28, 2012 RE: Conversion
2019 Transition Policy and Procedure
 2019 Transition Policy and Procedure POLICY Steward Health Choice Generations (SHCG) provides a Part D drug transition process in order to prevent enrollee medication coverage gaps. SHCG s transition process
2019 Transition Policy and Procedure POLICY Steward Health Choice Generations (SHCG) provides a Part D drug transition process in order to prevent enrollee medication coverage gaps. SHCG s transition process
Preferred IPA of California Claims Settlement Practices Provider Notification
 Preferred IPA of California Claims Settlement Practices Provider Notification As required by Assembly Bill 1455, the California Department of Managed Health Care has set forth regulations establishing
Preferred IPA of California Claims Settlement Practices Provider Notification As required by Assembly Bill 1455, the California Department of Managed Health Care has set forth regulations establishing
Alabama Medicaid Pharmacist
 Alabama Medicaid Pharmacist Published Quarterly by Health Information Designs, Inc., Fall 2005 A Service of Alabama Medicaid Medicare Modernization Act Adopted in December 2003, the Medicare Modernization
Alabama Medicaid Pharmacist Published Quarterly by Health Information Designs, Inc., Fall 2005 A Service of Alabama Medicaid Medicare Modernization Act Adopted in December 2003, the Medicare Modernization
Texas Vendor Drug Program. Drug Addition Process. Effective Date. December 2017
 Texas Vendor Drug Program Drug Addition Process Effective Date December 2017 This is a working document to provide a resource to interested internal and external stakeholders. Questions or comments regarding
Texas Vendor Drug Program Drug Addition Process Effective Date December 2017 This is a working document to provide a resource to interested internal and external stakeholders. Questions or comments regarding
RE: Comment on CMS-9937-P ( Patient Protection and Affordable Care Act; HHS Notice of Benefit and Payment Parameters for 2017: Proposed Rule )
 December 21, 2015 Centers for Medicare and Medicaid Services Department of Health and Human Services Hubert H. Humphrey Building, Room 445-G 200 Independence Avenue, SW Washington, D.C. 20201 RE: Comment
December 21, 2015 Centers for Medicare and Medicaid Services Department of Health and Human Services Hubert H. Humphrey Building, Room 445-G 200 Independence Avenue, SW Washington, D.C. 20201 RE: Comment
Reinsurance Fees Examples of Counting Methods
 Brought to you by Sullivan Benefits Reinsurance Fees Examples of Counting Methods The Affordable Care Act (ACA) created a transitional reinsurance program to help stabilize premiums in the individual market
Brought to you by Sullivan Benefits Reinsurance Fees Examples of Counting Methods The Affordable Care Act (ACA) created a transitional reinsurance program to help stabilize premiums in the individual market
The Merck Access Program ENROLLMENT FORM
 The Merck Access Program ENROLLMENT FORM P: 877-709-4455 F: 800-977-1957 The Merck Access Program, PO Box 29067, Phoenix, AZ 85038 TO GET STARTED, COMPLETE THE ENROLLMENT FORM AND FAX IT TO 800-977-1957.
The Merck Access Program ENROLLMENT FORM P: 877-709-4455 F: 800-977-1957 The Merck Access Program, PO Box 29067, Phoenix, AZ 85038 TO GET STARTED, COMPLETE THE ENROLLMENT FORM AND FAX IT TO 800-977-1957.
AGENCY: Internal Revenue Service, Department of the Treasury; Employee Benefits Security
 This document is scheduled to be published in the Federal Register on 07/22/2016 and available online at http://federalregister.gov/a/2016-17242, and on FDsys.gov DEPARTMENT OF THE TREASURY Internal Revenue
This document is scheduled to be published in the Federal Register on 07/22/2016 and available online at http://federalregister.gov/a/2016-17242, and on FDsys.gov DEPARTMENT OF THE TREASURY Internal Revenue
2008 Medicare Part D: Pharmacist's Survival Guide. Ronnie DePue, R.Ph., CGP
 2008 Medicare Part D: Pharmacist's Survival Guide Ronnie DePue, R.Ph., CGP Objectives At the completion of this program, the participant will be able to: 1. Give an overview of the Medicare Prescription
2008 Medicare Part D: Pharmacist's Survival Guide Ronnie DePue, R.Ph., CGP Objectives At the completion of this program, the participant will be able to: 1. Give an overview of the Medicare Prescription
340B Program Update & Recommendations for Monitoring Program Compliance October
 340B Program Update & Recommendations for Monitoring Program Compliance October 2 2014 Speaker Biography Ray Albertina Director Deloitte & Touche LLP +1 (314) 342 4984 ralbertina@deloitte.com Ray is a
340B Program Update & Recommendations for Monitoring Program Compliance October 2 2014 Speaker Biography Ray Albertina Director Deloitte & Touche LLP +1 (314) 342 4984 ralbertina@deloitte.com Ray is a
Re: Medicare Program; Reporting and Returning of Overpayments, CMS-6037-P, RIN 0938-AQ58, Federal Register, Thursday, February 16, 2012.
 Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-6037-P Mail Stop C4-26-05 7500 Security Boulevard Baltimore, MD 21244-1850 Re: Medicare Program; Reporting
Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS-6037-P Mail Stop C4-26-05 7500 Security Boulevard Baltimore, MD 21244-1850 Re: Medicare Program; Reporting
01/07/2014 Medicare Part D Coverage Gap Discount Program Program Dates Page 1 of 7
 01/07/2014 Medicare Part D Coverage Gap Discount Program Program Dates Page 1 of 7 and A. COVERAGE GAP DISCOUNT PROGRAM - BY CALENDAR YEAR 2011 through 2017 PDE Reporting Period Paid By After Receipt After
01/07/2014 Medicare Part D Coverage Gap Discount Program Program Dates Page 1 of 7 and A. COVERAGE GAP DISCOUNT PROGRAM - BY CALENDAR YEAR 2011 through 2017 PDE Reporting Period Paid By After Receipt After
Medicare Plan Payment Group. Date: August 8, All Part D Plan Sponsors, including PACE Organizations
 DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Center for Medicare 7500 Security Boulevard, Mail Stop C1-13-07 Baltimore, Maryland 21244-1850 Medicare Plan Payment Group
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Center for Medicare 7500 Security Boulevard, Mail Stop C1-13-07 Baltimore, Maryland 21244-1850 Medicare Plan Payment Group
REQUEST FOR MEDICARE PRESCRIPTION DRUG COVERAGE DETERMINATION This form may be sent to us by mail or fax:
 REQUEST FOR MEDICARE PRESCRIPTION DRUG COVERAGE DETERMINATION This form may be sent to us by mail or fax: Address: BlueCross BlueShield of Western New York P.O. Box 80 Buffalo, NY 14204 Attn: Pharmacy
REQUEST FOR MEDICARE PRESCRIPTION DRUG COVERAGE DETERMINATION This form may be sent to us by mail or fax: Address: BlueCross BlueShield of Western New York P.O. Box 80 Buffalo, NY 14204 Attn: Pharmacy
Agency Information Collection Activities: Proposed Collection; Comment Request
 This document is scheduled to be published in the Federal Register on 10/01/2018 and available online at https://federalregister.gov/d/2018-20995, and on govinfo.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
This document is scheduled to be published in the Federal Register on 10/01/2018 and available online at https://federalregister.gov/d/2018-20995, and on govinfo.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
Arkansas State University System Prescription Drug Program
 Arkansas State University System Prescription Drug Program The Arkansas State University (ASU) prescription drug program involves a partnership with the University of Arkansas for Medical Sciences (UAMS)
Arkansas State University System Prescription Drug Program The Arkansas State University (ASU) prescription drug program involves a partnership with the University of Arkansas for Medical Sciences (UAMS)
CMS released the 2018 Physician Fee Schedule Final Rule last week. The following is a summary of the AHRA-related policies.
 CMS released the 2018 Physician Fee Schedule Final Rule last week. The following is a summary of the AHRA-related policies. 1. Appropriate Use Criteria Delayed Until 2020 CMS had already proposed to delay
CMS released the 2018 Physician Fee Schedule Final Rule last week. The following is a summary of the AHRA-related policies. 1. Appropriate Use Criteria Delayed Until 2020 CMS had already proposed to delay
Memorandum. To: HCRRC From: Jayson Slotnik Date: Re: Summary of Outpatient Prospective Payment System Final Rule
 Memorandum To: HCRRC From: Jayson Slotnik Date: 11.4.2004 Re: Summary of Outpatient Prospective Payment System Final Rule On November 15, 2004, CMS will publish its final rule entitled, Medicare Program;
Memorandum To: HCRRC From: Jayson Slotnik Date: 11.4.2004 Re: Summary of Outpatient Prospective Payment System Final Rule On November 15, 2004, CMS will publish its final rule entitled, Medicare Program;
January 16, Submitted electronically via:
 Submitted electronically via: http://www.regulations.gov The Honorable Seema Verma, Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-4182-P
Submitted electronically via: http://www.regulations.gov The Honorable Seema Verma, Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-4182-P
July 23, RE: Comments on the Conversion of Net Income Standards to Equivalent Modified Adjusted Gross Income Standards. Dear Ms.
 July 23, 2012 Stephanie Kaminsky Center for Medicaid and CHIP Services Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services RE: Comments on the Conversion of Net Income
July 23, 2012 Stephanie Kaminsky Center for Medicaid and CHIP Services Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services RE: Comments on the Conversion of Net Income
NCPA summary of Part D Final Rule for contract year NCPA advocacy at work
 NCPA summary of Part D Final Rule for contract year 2019 The Centers for Medicare and Medicaid Services ( CMS ) recently issued its final rule (the Final Rule ) to revise the Medicare Advantage and Part
NCPA summary of Part D Final Rule for contract year 2019 The Centers for Medicare and Medicaid Services ( CMS ) recently issued its final rule (the Final Rule ) to revise the Medicare Advantage and Part
Medicare Advantage Part D Pharmacy Policy
 Page 1 of 27 DISCLAIMER NOTICE: The purpose of this policy is to provide guidance for benefit and coverage determinations only. Benefit and coverage determinations are subject to the contractual limitations
Page 1 of 27 DISCLAIMER NOTICE: The purpose of this policy is to provide guidance for benefit and coverage determinations only. Benefit and coverage determinations are subject to the contractual limitations
Prime Perspective. From the auditor s desk. Quarterly Pharmacy Newsletter from Prime Therapeutics LLC INSIDE. September 2018: Issue 73
 Prime Perspective Quarterly Pharmacy Newsletter from Prime Therapeutics LLC September 2018: Issue 73 From the auditor s desk INSIDE From the auditor s desk...1 Medicare news/ Medicaid news...2 HCSC news...4
Prime Perspective Quarterly Pharmacy Newsletter from Prime Therapeutics LLC September 2018: Issue 73 From the auditor s desk INSIDE From the auditor s desk...1 Medicare news/ Medicaid news...2 HCSC news...4
Draft Released: February 1, Final Released: April 2, Effective Date: January 1, 2019
 AMCP Summary: Announcement of Calendar Year (CY) 2019 Medicare Advantage Capitation Rates and Medicare Advantage and Part D Payment Policies and Final Call Letter Draft Released: February 1, 2018 Final
AMCP Summary: Announcement of Calendar Year (CY) 2019 Medicare Advantage Capitation Rates and Medicare Advantage and Part D Payment Policies and Final Call Letter Draft Released: February 1, 2018 Final
March 2, Dear Acting Administrator Tavenner:
 Marilyn Tavenner, Acting Administrator Center for Medicare and Medicaid Services (CMS) 7500 Security Boulevard C1-13-07 Baltimore, Maryland 21244 Re: Advance Notice of Methodological Changes for Calendar
Marilyn Tavenner, Acting Administrator Center for Medicare and Medicaid Services (CMS) 7500 Security Boulevard C1-13-07 Baltimore, Maryland 21244 Re: Advance Notice of Methodological Changes for Calendar
April 10, Major General Elder Granger Deputy Director, TMA Skyline Five, Suite Leesburg Pike Falls Church, VA
 Major General Elder Granger Deputy Director, TMA Skyline Five, Suite 810 5111 Leesburg Pike Falls Church, VA 22041-3206 Re: Dear Manufacturer Letter Dated February 1, 2008 Dear : The Biotechnology Industry
Major General Elder Granger Deputy Director, TMA Skyline Five, Suite 810 5111 Leesburg Pike Falls Church, VA 22041-3206 Re: Dear Manufacturer Letter Dated February 1, 2008 Dear : The Biotechnology Industry
Chapter 9: What to do if you have a problem or complaint (coverage decisions, appeals, complaints)
 Chapter 9: What to do if you have a problem or complaint (coverage decisions, appeals, complaints) SECTION 6 Your Part D prescription drugs: How to ask for a coverage decision or make an appeal? Have you
Chapter 9: What to do if you have a problem or complaint (coverage decisions, appeals, complaints) SECTION 6 Your Part D prescription drugs: How to ask for a coverage decision or make an appeal? Have you
February 19, Patient Protection and Affordable Care Act; HHS Notice of Benefit and Payment Parameters for 2020
 February 19, 2019 Submitted electronically via http://www.regulations.gov Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-9926-P P.O. Box 8016 Baltimore,
February 19, 2019 Submitted electronically via http://www.regulations.gov Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-9926-P P.O. Box 8016 Baltimore,
Center for Medicaid, CHIP, and Survey & Certification SMDL# PPACA# 2. April 22, Re: Medicaid Prescription Drug Rebates
 DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard, Mail Stop S2-26-12 Baltimore, Maryland 21244-1850 Center for Medicaid, CHIP, and Survey & Certification
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard, Mail Stop S2-26-12 Baltimore, Maryland 21244-1850 Center for Medicaid, CHIP, and Survey & Certification
Supporting Appropriate Payer Coverage Decisions
 Supporting Appropriate Payer Coverage Decisions Providing Services for Janssen Pharmaceutical Companies of Johnson & Johnson Table of Contents Introduction 3 This document is presented for informational
Supporting Appropriate Payer Coverage Decisions Providing Services for Janssen Pharmaceutical Companies of Johnson & Johnson Table of Contents Introduction 3 This document is presented for informational
RELIEF FOR ELIGIBLE PROFESSIONALS? PROPOSED STAGE 2 MEANINGFUL USE RULE INCLUDES IMPORTANT (POTENTIAL) EXCEPTIONS [OBER KALER]
![RELIEF FOR ELIGIBLE PROFESSIONALS? PROPOSED STAGE 2 MEANINGFUL USE RULE INCLUDES IMPORTANT (POTENTIAL) EXCEPTIONS [OBER KALER] RELIEF FOR ELIGIBLE PROFESSIONALS? PROPOSED STAGE 2 MEANINGFUL USE RULE INCLUDES IMPORTANT (POTENTIAL) EXCEPTIONS [OBER KALER]](/thumbs/88/116116176.jpg) RELIEF FOR ELIGIBLE PROFESSIONALS? PROPOSED STAGE 2 MEANINGFUL USE RULE INCLUDES IMPORTANT (POTENTIAL) EXCEPTIONS Publication RELIEF FOR ELIGIBLE PROFESSIONALS? PROPOSED STAGE 2 MEANINGFUL USE RULE INCLUDES
RELIEF FOR ELIGIBLE PROFESSIONALS? PROPOSED STAGE 2 MEANINGFUL USE RULE INCLUDES IMPORTANT (POTENTIAL) EXCEPTIONS Publication RELIEF FOR ELIGIBLE PROFESSIONALS? PROPOSED STAGE 2 MEANINGFUL USE RULE INCLUDES
Ref: CMS-2399-P: Medicaid Program; Disproportionate Share Hospital Payments Treatment of Third-Party Payers in Calculating Uncompensated Care Costs
 September, 14 2016 Mr. Andrew Slavitt Acting Administrator Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services Hubert H. Humphrey Building, Room 445-G 200 Independence
September, 14 2016 Mr. Andrew Slavitt Acting Administrator Centers for Medicare & Medicaid Services U.S. Department of Health and Human Services Hubert H. Humphrey Building, Room 445-G 200 Independence
Highlights from the proposed rule include the following:
 Proposed Physician Fee Schedule for CY 2011: Initial Summary of Issues of Concern to ASCO Members On June 25, 2010, the Centers for Medicare and Medicaid Services (CMS) displayed the proposed rule for
Proposed Physician Fee Schedule for CY 2011: Initial Summary of Issues of Concern to ASCO Members On June 25, 2010, the Centers for Medicare and Medicaid Services (CMS) displayed the proposed rule for
Common Managed Care Terms & Definitions
 Contact Us: Email: info@emedbiz.com Phone: 561-430-2090 Fax: 561-430-2091 Website: www.emedbiz.com Common Managed Care Terms & Definitions Balance billing: The practice of billing a patient for the amount
Contact Us: Email: info@emedbiz.com Phone: 561-430-2090 Fax: 561-430-2091 Website: www.emedbiz.com Common Managed Care Terms & Definitions Balance billing: The practice of billing a patient for the amount
Released: November 16, Comments Due: January 16, 2018
 AMCP Summary: Medicare Program; Contract Year 2019 Policy and Technical Changes to the Medicare Advantage, Medicare Cost Plan, Medicare Fee-for-Service, the Medicare Prescription Drug Benefit Programs,
AMCP Summary: Medicare Program; Contract Year 2019 Policy and Technical Changes to the Medicare Advantage, Medicare Cost Plan, Medicare Fee-for-Service, the Medicare Prescription Drug Benefit Programs,
Exclusion of Orphan Drugs for Certain Covered Entities under 340B Program
 Billing Code: 4165-15 DEPARTMENT OF HEALTH AND HUMAN SERVICES 42 CFR Part 10 RIN 0906- AA94 Exclusion of Orphan Drugs for Certain Covered Entities under 340B Program AGENCY: Health Resources and Services
Billing Code: 4165-15 DEPARTMENT OF HEALTH AND HUMAN SERVICES 42 CFR Part 10 RIN 0906- AA94 Exclusion of Orphan Drugs for Certain Covered Entities under 340B Program AGENCY: Health Resources and Services
