Update. The authors of this article are all consultants with Huron Consulting Group, which serves the continuum of life sciences organizations
|
|
|
- Edgar Skinner
- 6 years ago
- Views:
Transcription
1 Life Science Compliance Update REPRINTED FROM U.S. EDITION Volume 2.1 February 2016 Your Special Relationships Specialty Pharmacies and 5 Thoughtful Controls to Consider public advocates, and the media are now focusing more on initiatives and relationships between pharmaceutical companies, their distribution channels, other healthcare providers and patients. As pharmaceutical companies continually work to assist healthcare providers and patients in gaining by Jack Tanselle - Managing Director, John Moose - Director, Samantha Sutherland - Associate and Mark DeQyngaert - Managing Director from Huron Consulting Group The Government and other regulators are now focusing more on initiative relationships between pharmaceutical companies and other customer entities, beyond those relationships with individual healthcare professionals (HCPs). One area of increased focus involves specialty pharmacies. This article explores those relationships and suggests After years of focusing on the pharmaceutical industry s relationships with physicians, including dozens of Corporate Integrity Agreements (CIAs) centered on alleged off-label promotion or potential kickback arrangements, Government regulators, What is a Specialty Pharmacy? According to the American Pharmacist Association, a [s]pecialty pharmacy focuses on high cost, high touch medication therapy for patients with complex disease states. Medications in specialty pharmacy range from oral to cutting edge injectable and biologic products. The disease states treated range from cancer, multiple sclerosis, and rheumatoid arthritis to rare genetic conditions. The authors of this article are all consultants with Huron Consulting Group, which serves the continuum of life sciences organizations reduce the risks associated with regulatory and government scrutiny. Views expressed in this article are that of the authors and not necessarily those of Huron Consulting Group, or its clients, and should not be interpreted as legal advice. If you have questions about specialty pharmacy relationships or any other considerations, please feel free to contact Jack (317) ; jtanselle@ huronconsultinggroup.com or Mark (646) ; mdewyngaert@huronconsultinggroup.com 1
2 Specialty Pharmacy Risks The list below details several of the risks that have been highlighted in recent settlements and media coverage. 1. Specialty pharmacies might fill a prescription for a branded product of a pharmaceutical company with whom they have a contractual and financial relationship, without providing or making available to the patient balanced information and less expensive alternatives. 2. Specialty pharmacies may distribute products for only one pharmaceutical company; with the pharmaceutical company representatives encouraging physicians to have patients only fill prescriptions through that one specialty pharmacy. 3. Pharmaceutical companies may get too deeply involved in the claims/copay adjudication process associated with fulfilling prescriptions, including potentially altering the physician s orders, as a way of reducing the administrative burden on physicians and patients. 4. Pharmaceutical companies may come to rely upon specialty pharmacies for products that do not meet the generally accepted criteria for such special handling and patient care. access to drugs (e.g., drug samples, vouchers, copay cards, patient assistance programs), specialty pharmacies have grown in quantity and importance. The growth in accredited specialty pharmacy locations is significant with fewer than 20 in 2009 and now as many as 250 at the end of While patient demographics and product portfolios at many pharmaceutical companies align with this increase in specialty pharmacies, recent allegations, and media scrutiny during the latter half of 2015, suggest that some of the increase in the use of the specialty distribution channel may be perceived as an inappropriate driver for unnecessary drug utilization. Potential Risks Posed by Relationships with Specialty Pharmacies With the increased use of specialty pharmacies, there has been a corresponding increase in the potential risks associated with this distribution model. Some of these risks are inherent to any relationship within the pharmaceutical industry. However, certain risks are specific to this type of relationship. Many specialty pharmacies are incentivized through metrics involving volume thresholds or share of the market, typically in the form of adherence or refill prescription targets agreed upon with the manufacturer. These thresholds may incentivize the pharmacy to manipulate the information provided to the patient (i.e. alternative therapy options, misleading or removal of key product information, etc.) and ultimately can result in over-utilization. Recent litigation shows this is a growing concern and has resulted in false claims being submitted to federal health care programs. 2 2
3 Additionally, there is a high risk for rejection of lower cost alternative therapies by the patient due to the manufacturer and specialty pharmacy relationship. Potential lower-cost alternative therapies can be in the form of a generic product or a lower-cost brand alternative. This is a growing concern, with an emphasis on lowering prescription costs as a whole in the healthcare landscape. Specialty pharmacies can be appropriately incentivized to place adherence communication calls as part of operating disease-management programs to improve clinical outcomes. Agreements with the pharmacies that specifically preclude informing patients of generic or lower-cost brand alternatives, with a similar efficacy as the specialty product they have previously been prescribed, may present a conflict of interest risk. The claims/co-pay adjudication process often is outsourced to third parties or handled internally by major pharmaceutical companies. Patients are referred to a division called Patient Services, with the goal being to make sure a patient receives, and stays, on the appropriate drug as set by the patient s physician. The legitimate intent of the pharmaceutical company is to provide assistance to patients and make it easier for them to access therapy. However, such actions also come with the potential risk of perceived or real conflict of interests for two possible reasons. First, offering too much assistance to any customer (e.g., specialty pharmacy, patient, physician), without receiving compensation for it, might be perceived as a kickback. Offering assistance may also lead to perceptions of driving an unnecessary increase in the utilization of your branded products, which in turn could lead to allegations associated with the False Claims Act. 5 Controls to Consider To better manage risks within your company, there are controls that can be implemented to help account for existing and future activities. One such external control is already occurring as it relates to these specialty pharmacy operations. Leading pharmacy benefit managers have started excluding certain specialty pharmacies called out by recent media attention, thereby cutting off this pathway downstream from the patient and physician. The key question to ask within your organization is what controls does your company have in place to help mitigate these risks with specialty pharmacy relationships, or with identifying the next risk that emerges in the never-ending search for creative approaches to improving product development and patient care. For specialty pharmacy relationships, below are five controls your company should consider to potentially address current and future risks. 1. Justify the Need: Assess current specialty pharmacy relationships and establish or update standard processes to help ensure that each arrangement with any customer can be appropriately justified. The industry has come to understand the concept of a needs assessment, that was first included in the CIA s with several of the world s leading orthopedic manufacturers. Subsequent settlements with pharmaceutical manufacturers have included similar requirements, whereby at an aggregate-level annually (e.g., annual needs assessment), and per each activity (e.g., 3
4 rationale documents), a company assesses the actual need for each customer arrangement, as well as the type and volume of HCPs involved, and frequency of the activity, to be sure there is a legitimate business need prior to commencing the engagement. 3 Based on this idea, one immediate action to consider is assessing the legitimate business needs associated with each specialty pharmacy relationship and the services your company is requesting to be performed. In conducting such an assessment, first determine if the products involved in such relationships warrant the special handling or patient care involved with the use of specialty pharmacies (see definition provided earlier). Also, assess the requirements of both parties outlined in each specialty pharmacy contract and determine if either party is required to perform activities, such as those outlined earlier, that may be creating unnecessary risk for your company, the specialty pharmacy, or both. On a go-forward basis, it is important to justify each customer engagement and manage that justification throughout the life of the contractual terms. With proposed and recent changes from the Centers for Medicare and Medicaid Services (CMS) regarding itemization and scrutiny from a variety of regulatory agencies, companies may want to reevaluate how they are determining the need for, negotiating, and drafting their thirdparty distribution agreements to reflect properly the specific activities the third parties, or your company, will be performing, the metrics for measurement, and the expected deliverables that will support FMV payment for these services. 2. Catalog All Customer Activities: Include in your annual risk assessment a catalog of all of the different types of business arrangements your company has in place with all of the different types of customers, including specialty pharmacies, with which your company does business. In addition to assessing the legitimacy of any activity, the risks associated with conducting those activities should also be routinely assessed. As a participant in the U.S. Federal Health Care system, your company faces potential scrutiny for any type of arrangement you make with any entity involved in the prescribing or dispensing of your products. Your company likely has numerous types of existing arrangements with prescribers and dispensers of your products, and those individuals in your company involved in commercializing products will continually be challenged with creating new types of arrangements going forward that support improved healthcare delivery. Does your company have an existing catalog or database of all such customer activities to leverage in an ongoing manner as part of your current and future risk assessment process? While there are multiple facets to a strong riskassessment process, successfully summarizing the triangle of company products, customers (e.g., physicians, specialty pharmacies, other distributors), and associated business arrangements is a critical first step. Each time a risk assessment process begins (e.g., annually), the summary should be revisited and updated based on input from commercial, medical, clinical, financial, legal and compliance colleagues. This does not guarantee risk prevention, but not maintaining such a list may greatly increase the 4
5 chances that you find out about such risks when it is too late to mitigate them quickly. 3. Justify the Value: Establish or update standard processes to help ensure that fair market value (FMV) is being paid for any bona fide services specialty pharmacies might be providing to your company as part of any contract or engagement. In addition to justifying the conduct of any activity with a customer, it is also critical to justify the value of the specialty pharmacy arrangement and to be sure that your company has documented policies and procedures that are followed to determine the fair market value of all such arrangements. There is a further consideration regarding the bona fide nature of the services that may impact government price reporting calculations. Consider a review of the payment process as well, to ensure that contracted services were actually performed. It is also important to assess whether your company is directly or indirectly providing additional services to customers, such as getting involved in the claims/co-pay adjudication process, as described earlier (i.e., helping providers and patients with completing paperwork or assisting with clearing prior authorization hurdles associated with many healthcare plans). In order to mitigate the risk of conducting activities that may be perceived as kickbacks, your company should consider conducting a careful analysis of exactly what types of support are being provided and the impact on third-party payers. 4. Other In-Process Controls: Consider implementing other specific controls to manage off-label and anti-kickback risks (e.g., reviewing compensation plans, utilizing inclusion/exclusion lists). The CIAs of the last decade provide guidance to some specific controls that can potentially be applied to your specialty pharmacy relationships. For example, for companies assisting with prior authorization and other patient support services, either directly or through a third party like a specialty pharmacy, any compensation plans for those employees or agents of the company should be closely reviewed and approved by your company s Legal department on an annual basis. If leveraging a third party, contract terms should consider your company s right to review compensation plans as one of your company s controls in the overall effort to mitigate risk with specialty pharmacy relationships. Just as sales representatives should not be incentivized to sell to physicians whose specialties do not align with the indication(s) on product label(s), scrutiny should be given to the idea of any employee or agent of a company being incentivized on the volume of patients receiving reimbursement assistance and clearance for their company s drugs. That volume should be driven by physician prescriptions to their patients. Specialty pharmacies should also potentially be evaluated with an inclusion/exclusion list, akin to those used by companies under CIAs in order to control the specialties sales representatives call on and provide samples to. In the case of specialty pharmacies, such a list would indicate 5
6 any products in the portfolio that may not warrant special care or attention to go through that channel. In having such a list, individuals involved in the approval processes for various contracts with specialty pharmacies and other customers can be trained to help assess for such misalignment and stop such agreements from being executed in the first place. 5. Audit and Monitor: Consistently include in your company s auditing and monitoring activities those activities that cover the full range of products, customers, and activities? The first four controls can help mitigate risk because they are controls built into the processes at the earliest stages of creating various business activities. Compliance auditing and monitoring is another control that allows for assessing risk as activities are taking place, or after they have taken place. If your company has specialty pharmacy relationships, and you are uncertain about the risks associated with them, auditing one or more of those contracts should give you an indication of the risks, either at the level of one or more of the specific specialty pharmacies, and/or your company s systemic processes for establishing and managing those relationships. The auditing and monitoring plan should be derived annually from the risk assessment output, such that identifying new activities that carry risks untested should then be included in the subsequent year s auditing and monitoring plan for more in-depth review. In turn, the output from a quality auditing and monitoring program should be used as input to the next cycle of risk assessment, such that a virtuous cycle is created and maintained to contribute to the continuous improvement that any effective compliance program will demonstrate. Conclusion Acting on some or all of the recommended controls outlined in this article should allow your company to mitigate better risks associated with specialty pharmacy relationships. Understanding that new business activities and arrangements will be created in the future, creating new risks, it is important to consider the controls outlined in this article for more than just managing specialty pharmacy relationships. Specifically, the fundamentals involved with managing an effective compliance program should not change because the customer activities conducted by commercial, R&D or medical affairs departments evolve over time. If companies are not implementing consistent risk assessment processes designed to ask questions across the entire customer continuum Summary: 5 Controls to Consider 1. Justify the need 2. Catalog all customer activities 3. Justify the value 4. Other in-process controls 5. Audit and monitor 6
7 (i.e., all activities conducted with all customers for all brands), as well as conducting appropriate monitoring controls throughout the lifecycle of each customer relationship (i.e., from strategic and budgeting planning to payment and reporting), the next creative, yet questionable, approach to managing this hyper-competitive and highly regulated marketplace may surface within your until it is too late. Copyright 2016, Life Science Compliance Update This publication may not be reproduced in any form without express consent of the publisher. Reprints of this publication can be obtained by contacting: Life Science Compliance Update Visit 1 See Deena Beasley, Specialty pharmacies in spotlight as Valeant ties questioned, Reuters (Oct. 22, 2015) at com/article/2015/10/22/us-valeant-pharmacies-industry-iduskcn 0SG #ApHjcXzoUZDh9TQk.97; see also Kaitlin Fallon, Specialty Pharma in the Spotlight Novartis Settlement Finalized, Life Science Compliance Update (Jan. 2016) at lifescicompliance.com. 2 See Tom Moylan, Another Specialty Pharmacy Settles Exjade False Claims Allegations For $45 Million, LexisNexis (May, ) at 3 See Calisha Myers, Now You See It, Now You Don t A Review of CIA Provisions from 2009 to 2015, Life Science Compliance Update (Jan. 2016) at Jack Tanselle jtanselle@huronconsultinggroup.com Mark DeWyngaert, PhD, MBA mdeqyngaert@huronconsultinggroup.com 7
Lindsey Imada, PharmD Candidate 2016 Midwestern University, Chicago College of Pharmacy
 Lindsey Imada, PharmD Candidate 2016 Midwestern University, Chicago College of Pharmacy Under the Preceptorship of Dr. Craig Stern Pro Pharma Pharmaceutical Consultants, Inc. September 11, 2015 S OBJECTIVES
Lindsey Imada, PharmD Candidate 2016 Midwestern University, Chicago College of Pharmacy Under the Preceptorship of Dr. Craig Stern Pro Pharma Pharmaceutical Consultants, Inc. September 11, 2015 S OBJECTIVES
COMPLIANCE WITH PATIENT ASSISTANCE PROGRAMS AND CO-PAY CARDS. Judd Katz JD MHA November 2016
 COMPLIANCE WITH PATIENT ASSISTANCE PROGRAMS AND CO-PAY CARDS Judd Katz JD MHA November 2016 Background information Patient Assistance Programs Copay Cards/Assistance Programs Reimbursement Support AGENDA
COMPLIANCE WITH PATIENT ASSISTANCE PROGRAMS AND CO-PAY CARDS Judd Katz JD MHA November 2016 Background information Patient Assistance Programs Copay Cards/Assistance Programs Reimbursement Support AGENDA
Structuring Specialty Pharmacy Distribution Arrangements in a Turbulent Regulatory Environment Mini Summit XVIII
 Structuring Specialty Pharmacy Distribution Arrangements in a Turbulent Regulatory Environment Mini Summit XVIII The 16 th Pharmaceutical Compliance Congress and Best Practices Forum Thursday, October
Structuring Specialty Pharmacy Distribution Arrangements in a Turbulent Regulatory Environment Mini Summit XVIII The 16 th Pharmaceutical Compliance Congress and Best Practices Forum Thursday, October
2017 PHARMACEUTICAL COMPLIANCE CONGRESS
 2017 PHARMACEUTICAL COMPLIANCE CONGRESS ASSESS EMERGING RISKS AND THE ROLE FOR COMPLIANCE ACROSS MARKET ACCESS ACTIVITIES APRIL 27, 2017 PRESENTERS JANE H. YOON Of Counsel Paul Hastings LLP JACK TANSELLE
2017 PHARMACEUTICAL COMPLIANCE CONGRESS ASSESS EMERGING RISKS AND THE ROLE FOR COMPLIANCE ACROSS MARKET ACCESS ACTIVITIES APRIL 27, 2017 PRESENTERS JANE H. YOON Of Counsel Paul Hastings LLP JACK TANSELLE
Contracting with Specialty Pharmacies and Hubs 17 th Annual Pharma and Medical Device Compliance Congress. October 20, 2016
 Contracting with Specialty Pharmacies and Hubs 17 th Annual Pharma and Medical Device Compliance Congress October 20, 2016 Thomas Beimers Hogan Lovells Thomas.beimers@hoganlovells.com Sarah Franklin Covington
Contracting with Specialty Pharmacies and Hubs 17 th Annual Pharma and Medical Device Compliance Congress October 20, 2016 Thomas Beimers Hogan Lovells Thomas.beimers@hoganlovells.com Sarah Franklin Covington
Specialty Pharmacies. Ensuring Compliant Relationships. April 2017
 Specialty Pharmacies Ensuring Compliant Relationships April 2017 Agenda I. Current climate II. Regulatory Overview III. Types of SPP relationships IV. Data purchase arrangements V. Fee for service arrangements
Specialty Pharmacies Ensuring Compliant Relationships April 2017 Agenda I. Current climate II. Regulatory Overview III. Types of SPP relationships IV. Data purchase arrangements V. Fee for service arrangements
Innovative Strategies for Managing the Rising Cost of Specialty Drugs
 Innovative Strategies for Managing the Rising Cost of Specialty Drugs Mid-sized Retirement and Healthcare Plan Management Conference Chicago, IL June 5, 2013 Managing the Rising Cost of Specialty Drugs
Innovative Strategies for Managing the Rising Cost of Specialty Drugs Mid-sized Retirement and Healthcare Plan Management Conference Chicago, IL June 5, 2013 Managing the Rising Cost of Specialty Drugs
The Management of Specialty Drugs: Opportunities and Challenges
 The Management of Specialty Drugs: Opportunities and Challenges Scott Woods Senior Director, Policy PCMA Innovations X April 5, 2016 Specialty Drugs to be Half of Spend by 2018 Forecast PMPM Net Drug
The Management of Specialty Drugs: Opportunities and Challenges Scott Woods Senior Director, Policy PCMA Innovations X April 5, 2016 Specialty Drugs to be Half of Spend by 2018 Forecast PMPM Net Drug
ACTIVELY MANAGED DRUG SOLUTIONS SPECIALTY DRUGS. Supporting employees and building sustainable drug plans...together
 ACTIVELY MANAGED DRUG SOLUTIONS SPECIALTY DRUGS Supporting employees and building sustainable drug plans...together Not available in the province of Quebec INTRODUCING THE SPECIALTY DRUG PROGRAM If you
ACTIVELY MANAGED DRUG SOLUTIONS SPECIALTY DRUGS Supporting employees and building sustainable drug plans...together Not available in the province of Quebec INTRODUCING THE SPECIALTY DRUG PROGRAM If you
CBI Pharmaceutical Compliance Congress Washington, D.C.
 Risks Associated with the Hub CBI Pharmaceutical Compliance Congress Washington, D.C. April 28, 2017 Disclaimer On behalf of this panel, please note that the views and opinions that will be expressed during
Risks Associated with the Hub CBI Pharmaceutical Compliance Congress Washington, D.C. April 28, 2017 Disclaimer On behalf of this panel, please note that the views and opinions that will be expressed during
A n area that has garnered considerable government
 Pharmaceutical Law & Industry Report Reproduced with permission from Pharmaceutical Law & Industry Report, 15 PLIR 13, 03/31/2017. Copyright 2017 by The Bureau of National Affairs, Inc. (800-372-1033)
Pharmaceutical Law & Industry Report Reproduced with permission from Pharmaceutical Law & Industry Report, 15 PLIR 13, 03/31/2017. Copyright 2017 by The Bureau of National Affairs, Inc. (800-372-1033)
CBI 4th Reimbursement and Contracting Conference: Key Challenges Related to Specialty Drug Pricing and Contracting
 CBI 4th Reimbursement and Contracting Conference: Key Challenges Related to Specialty Drug Pricing and Contracting Avalere Health An Inovalon Company February 28, 2017 Growth in Drug Costs Relative to
CBI 4th Reimbursement and Contracting Conference: Key Challenges Related to Specialty Drug Pricing and Contracting Avalere Health An Inovalon Company February 28, 2017 Growth in Drug Costs Relative to
RESPIRONICS, INC. CONTRACTING WITH HEALTHCARE PROFESSIONALS OR PROVIDERS AND REFERRAL SOURCES POLICY
 Page 1 of 6 RESPIRONICS, INC. CONTRACTING WITH HEALTHCARE PROFESSIONALS OR PROVIDERS AND REFERRAL SOURCES POLICY I. Purpose This document sets forth Respironics, Inc. s ( Company ) policy for engaging
Page 1 of 6 RESPIRONICS, INC. CONTRACTING WITH HEALTHCARE PROFESSIONALS OR PROVIDERS AND REFERRAL SOURCES POLICY I. Purpose This document sets forth Respironics, Inc. s ( Company ) policy for engaging
2018 Medicare Part D Transition Policy
 Regulation/ Requirements Purpose Scope Policy 2018 Medicare Part D Transition Policy 42 CFR 423.120(b)(3) 42 CFR 423.154(a)(1)(i) 42 CFR 423.578(b) Medicare Prescription Drug Benefit Manual, Chapter 6,
Regulation/ Requirements Purpose Scope Policy 2018 Medicare Part D Transition Policy 42 CFR 423.120(b)(3) 42 CFR 423.154(a)(1)(i) 42 CFR 423.578(b) Medicare Prescription Drug Benefit Manual, Chapter 6,
2008 Medicare Part D: Pharmacist's Survival Guide. Ronnie DePue, R.Ph., CGP
 2008 Medicare Part D: Pharmacist's Survival Guide Ronnie DePue, R.Ph., CGP Objectives At the completion of this program, the participant will be able to: 1. Give an overview of the Medicare Prescription
2008 Medicare Part D: Pharmacist's Survival Guide Ronnie DePue, R.Ph., CGP Objectives At the completion of this program, the participant will be able to: 1. Give an overview of the Medicare Prescription
KEEPING PRESCRIPTION DRUGS AFFORDABLE: The Value of Pharmacy Benefit Managers (PBMs)
 The Texas Association of Health Plans Representing health insurers, health maintenance organizations, and other related health care entities operating in Texas. KEEPING PRESCRIPTION DRUGS AFFORDABLE: The
The Texas Association of Health Plans Representing health insurers, health maintenance organizations, and other related health care entities operating in Texas. KEEPING PRESCRIPTION DRUGS AFFORDABLE: The
Challenges in High Dollar Drugs. Suzanne Francart, PharmD, BCPS Manager Infusion Services & Medication Assistance Program UNC HealthCare
 Challenges in High Dollar Drugs Suzanne Francart, PharmD, BCPS Manager Infusion Services & Medication Assistance Program UNC HealthCare Disclosure I have no relevant conflicts of interest to disclose Learning
Challenges in High Dollar Drugs Suzanne Francart, PharmD, BCPS Manager Infusion Services & Medication Assistance Program UNC HealthCare Disclosure I have no relevant conflicts of interest to disclose Learning
Arkansas State University System Prescription Drug Program
 Arkansas State University System Prescription Drug Program The Arkansas State University (ASU) prescription drug program involves a partnership with the University of Arkansas for Medical Sciences (UAMS)
Arkansas State University System Prescription Drug Program The Arkansas State University (ASU) prescription drug program involves a partnership with the University of Arkansas for Medical Sciences (UAMS)
Pharmaceutical Management Community Plans 2018
 Pharmaceutical Management Community Plans 2018 Customer Service: (888) 327-0671 TTY: 711 Pharmacy Administration: (810) 244-1660 Introduction Pharmaceutical management promotes the use of the most clinically
Pharmaceutical Management Community Plans 2018 Customer Service: (888) 327-0671 TTY: 711 Pharmacy Administration: (810) 244-1660 Introduction Pharmaceutical management promotes the use of the most clinically
Prescription Drug Coverage
 The Company s medical plans automatically include coverage for prescription drugs which is administered by Envision Pharmaceutical Services, Inc. (Envision Rx) for prescriptions filled at retail pharmacies
The Company s medical plans automatically include coverage for prescription drugs which is administered by Envision Pharmaceutical Services, Inc. (Envision Rx) for prescriptions filled at retail pharmacies
The Declining Value of Payer Access: Defining and improving Rebate Efficiency in the current healthcare landscape
 The Declining Value of Payer Access: Defining and improving Rebate Efficiency in the current healthcare landscape Lucas Greenwalt, Senior Principal Amundsen Consulting Prepared for: CBI Gross to Net Boot
The Declining Value of Payer Access: Defining and improving Rebate Efficiency in the current healthcare landscape Lucas Greenwalt, Senior Principal Amundsen Consulting Prepared for: CBI Gross to Net Boot
Glossary of Terms (Terms are listed in Alphabetical Order)
 Glossary of Terms (Terms are listed in Alphabetical Order) Access Access refers to the availability and location of pharmacies that participate in the network that serves your pharmacy benefit plan. Acute
Glossary of Terms (Terms are listed in Alphabetical Order) Access Access refers to the availability and location of pharmacies that participate in the network that serves your pharmacy benefit plan. Acute
21 - Pharmacy Services
 21 - Pharmacy Services The role of Health Plan of Nevada s (HPN) Pharmacy Services is to evaluate and determine the appropriateness of quality drug therapy while maintaining and improving therapeutic outcomes.
21 - Pharmacy Services The role of Health Plan of Nevada s (HPN) Pharmacy Services is to evaluate and determine the appropriateness of quality drug therapy while maintaining and improving therapeutic outcomes.
Track III-A. Creating Relationships with Prescription Drug Plans and Managed Care Organizations
 Track III-A Creating Relationships with Prescription Drug Plans and Managed Care Organizations David L. Ralston Legal Director Schering-Plough Corporation Part D is for Dollars Follow the money by tracing
Track III-A Creating Relationships with Prescription Drug Plans and Managed Care Organizations David L. Ralston Legal Director Schering-Plough Corporation Part D is for Dollars Follow the money by tracing
Chapter 10 Prescriptions Benefits and Drug Formulary
 10 Prescription Benefits and Drug Formulary Health Choice Generations is a Medicare Advantage Special Needs Plan (SNP) with Medicare Part D Prescription Drug Coverage. Medicare Part D drugs covered by
10 Prescription Benefits and Drug Formulary Health Choice Generations is a Medicare Advantage Special Needs Plan (SNP) with Medicare Part D Prescription Drug Coverage. Medicare Part D drugs covered by
Moving From Offers to Solutions
 Moving From Offers to Solutions ALIGN CHANNEL STRATEGIES WITH PATIENT NEEDS TO REDUCE ACCESS BARRIERS Doug Gabbard The views and opinions expressed and presented here are my own and do not reflect the
Moving From Offers to Solutions ALIGN CHANNEL STRATEGIES WITH PATIENT NEEDS TO REDUCE ACCESS BARRIERS Doug Gabbard The views and opinions expressed and presented here are my own and do not reflect the
Access, Quality & Transparency: The Forgotten Issues in the Healthcare Debate Presented at WCIF Benefits Summit April 19, 2017
 Access, Quality & Transparency: The Forgotten Issues in the Healthcare Debate Presented at WCIF Benefits Summit April 19, 2017 What s happened? What s next? The ACA remains the Law of the Land for now!
Access, Quality & Transparency: The Forgotten Issues in the Healthcare Debate Presented at WCIF Benefits Summit April 19, 2017 What s happened? What s next? The ACA remains the Law of the Land for now!
Blue Essentials, Blue Advantage HMO SM and Blue Premier SM Provider Manual - Pharmacy
 Blue Essentials, Blue Advantage HMO SM and Blue Premier SM Provider Manual - In this Section there are references unique to Blue Essentials, Blue Advantage HMO and Blue Premier. These network specific
Blue Essentials, Blue Advantage HMO SM and Blue Premier SM Provider Manual - In this Section there are references unique to Blue Essentials, Blue Advantage HMO and Blue Premier. These network specific
A Special Type of Government Scrutiny: Pharmaceutical Manufacturer Relationships with Specialty Pharmacies: Part II
 April 2017 Follow @Paul_Hastings A Special Type of Government Scrutiny: Pharmaceutical Manufacturer Relationships with Specialty Pharmacies: Part II By Gary F. Giampetruzzi & Jonathan Stevens Reproduced
April 2017 Follow @Paul_Hastings A Special Type of Government Scrutiny: Pharmaceutical Manufacturer Relationships with Specialty Pharmacies: Part II By Gary F. Giampetruzzi & Jonathan Stevens Reproduced
Co-pay Card Program Monitoring and Optimization November 2014
 Primary/Final Payer Analysis Co-pay Card Program Monitoring and Optimization November 2014 Symphony Health Solutions offers an array of Managed Markets Studies CONSULTING/ANALYTICAL STUDIES Managed Markets
Primary/Final Payer Analysis Co-pay Card Program Monitoring and Optimization November 2014 Symphony Health Solutions offers an array of Managed Markets Studies CONSULTING/ANALYTICAL STUDIES Managed Markets
GERALD (JERRY) LEWANDOWSKI. BERKELEY RESEARCH GROUP, LLC 1800 M Street NW, Second Floor Washington, DC 20036
 Curriculum Vitae GERALD (JERRY) LEWANDOWSKI BERKELEY RESEARCH GROUP, LLC 1800 M Street NW, Second Floor Washington, DC 20036 Direct: 202.480.2643 Mobile: 202.258.2669 jlewandowski@thinkbrg.com Jerry Lewandowski
Curriculum Vitae GERALD (JERRY) LEWANDOWSKI BERKELEY RESEARCH GROUP, LLC 1800 M Street NW, Second Floor Washington, DC 20036 Direct: 202.480.2643 Mobile: 202.258.2669 jlewandowski@thinkbrg.com Jerry Lewandowski
Coverage Determinations, Appeals and Grievances
 Coverage Determinations, Appeals and Grievances Filing a grievance (making a complaint) about your prescription coverage Asking for a coverage determination (coverage decision) 60-day formulary change
Coverage Determinations, Appeals and Grievances Filing a grievance (making a complaint) about your prescription coverage Asking for a coverage determination (coverage decision) 60-day formulary change
DEPARTMENT OF HEALTH AND HUMAN SERVICES. Office of Inspector General s Use of Agreements to Protect the Integrity of Federal Health Care Programs
 United States Government Accountability Office Report to Congressional Requesters April 2018 DEPARTMENT OF HEALTH AND HUMAN SERVICES Office of Inspector General s Use of Agreements to Protect the Integrity
United States Government Accountability Office Report to Congressional Requesters April 2018 DEPARTMENT OF HEALTH AND HUMAN SERVICES Office of Inspector General s Use of Agreements to Protect the Integrity
Welcome. AMCP Partnership Forum. Designing Benefits and Payment Models for Innovative High Investment Medications
 AMCP Partnership Forum Designing Benefits and Payment Models for Innovative High Investment Medications Welcome Bri Palowitch, PharmD, BCGP Manager, Pharmacy Affairs Academy of Managed Care Pharmacy Disclaimer
AMCP Partnership Forum Designing Benefits and Payment Models for Innovative High Investment Medications Welcome Bri Palowitch, PharmD, BCGP Manager, Pharmacy Affairs Academy of Managed Care Pharmacy Disclaimer
SelectHealth Prescriptions
 SelectHealth Prescriptions pharmacy benefit management program SM SelectHealth Prescriptions is a full-service Pharmacy Benefit Manager (PBM) that offers transparent pricing, clinically based programs,
SelectHealth Prescriptions pharmacy benefit management program SM SelectHealth Prescriptions is a full-service Pharmacy Benefit Manager (PBM) that offers transparent pricing, clinically based programs,
Pharmacy Benefit Management in Oncology
 Pharmacy Benefit Management in Oncology October 28 th, 2015 Business Health Care Group Protecting the Future of Oncology Care: A Community Conversation Brent Eberle RPh MBA Chief Pharmacy Officer, Navitus
Pharmacy Benefit Management in Oncology October 28 th, 2015 Business Health Care Group Protecting the Future of Oncology Care: A Community Conversation Brent Eberle RPh MBA Chief Pharmacy Officer, Navitus
Understanding Your Prescription Program. CCIU Employee Meeting September 7, 2016
 Understanding Your Prescription Program CCIU Employee Meeting September 7, 2016 Welcome to FutureScripts! Founded in 2006 Philadelphia presence Strong ties to community and local businesses 68,000 pharmacies
Understanding Your Prescription Program CCIU Employee Meeting September 7, 2016 Welcome to FutureScripts! Founded in 2006 Philadelphia presence Strong ties to community and local businesses 68,000 pharmacies
Testimony of Mark Merritt. Pharmaceutical Care Management Association
 Testimony of Mark Merritt Pharmaceutical Care Management Association Before the UNITED STATES SENATE COMMITTEE ON HEALTH, EDUCATION, LABOR, AND PENSIONS The Cost of Prescription Drugs: How the Drug Delivery
Testimony of Mark Merritt Pharmaceutical Care Management Association Before the UNITED STATES SENATE COMMITTEE ON HEALTH, EDUCATION, LABOR, AND PENSIONS The Cost of Prescription Drugs: How the Drug Delivery
Get the most out of your pharmacy benefit.
 Get the most out of your pharmacy benefit. The ins and outs of managing pharmacy costs (and how the right information can lead to big savings). Learn more about the Artemis Platform at: artemishealth.com
Get the most out of your pharmacy benefit. The ins and outs of managing pharmacy costs (and how the right information can lead to big savings). Learn more about the Artemis Platform at: artemishealth.com
Managing Specialty Pharmaceuticals: Balancing Access and Affordability
 Managing Specialty Pharmaceuticals: Balancing Access and Affordability Commercial Health Plan Perspective The Health Industry Forum July 16, 2008 Presented by: Margaret M. (Peggy) Johnson, R.Ph. Vice President
Managing Specialty Pharmaceuticals: Balancing Access and Affordability Commercial Health Plan Perspective The Health Industry Forum July 16, 2008 Presented by: Margaret M. (Peggy) Johnson, R.Ph. Vice President
CWAG Prescription Drug Pricing Webinar
 CWAG Prescription Drug Pricing Webinar January 9, 2018 Kipp Snider, J.D. Vice President, State Policy Pharmaceutical Research & Manufacturers of America (PhRMA) Medicines Are Expected to Account for a
CWAG Prescription Drug Pricing Webinar January 9, 2018 Kipp Snider, J.D. Vice President, State Policy Pharmaceutical Research & Manufacturers of America (PhRMA) Medicines Are Expected to Account for a
ECONOMIC PRINCIPLES IMPACTING MANAGED CARE PHARMACY. Adrian Washington PharmD., MBA Vice President of Client Management United Healthcare OptumRx
 ECONOMIC PRINCIPLES IMPACTING MANAGED CARE PHARMACY Adrian Washington PharmD., MBA Vice President of Client Management United Healthcare OptumRx As vice president, Adrian is responsible for strategic planning
ECONOMIC PRINCIPLES IMPACTING MANAGED CARE PHARMACY Adrian Washington PharmD., MBA Vice President of Client Management United Healthcare OptumRx As vice president, Adrian is responsible for strategic planning
Manufacturer Patient Support Initiatives: Current Practices and Recent Challenges. Andrew Ruskin Morgan Lewis
 Intersecting Worlds of Drug, Device, Biologics and Health Law AHLA/FDLI May 22, 2012 Manufacturer Patient Support Initiatives: Current Practices and Recent Challenges by Andrew Ruskin Morgan Lewis The
Intersecting Worlds of Drug, Device, Biologics and Health Law AHLA/FDLI May 22, 2012 Manufacturer Patient Support Initiatives: Current Practices and Recent Challenges by Andrew Ruskin Morgan Lewis The
Pharmaceutical Management Medicaid 2017
 Pharmaceutical Management Medicaid 2017 Customer Service: (888) 327-0671 TTY: 711 Pharmacy Administration: (810) 244-1660 Visit our website at: McLarenHealthPlan.org MHP42721056 5/2017 Introduction Pharmaceutical
Pharmaceutical Management Medicaid 2017 Customer Service: (888) 327-0671 TTY: 711 Pharmacy Administration: (810) 244-1660 Visit our website at: McLarenHealthPlan.org MHP42721056 5/2017 Introduction Pharmaceutical
Industry Consolidation: Role of Compliance in Mergers, Acquisitions, and Divestitures
 Industry Consolidation: Role of Compliance in Mergers, Acquisitions, and Divestitures Prepared for CBI s Pharmaceutical Compliance Congress April 28, 2017 M&A Activity in the Pharmaceutical Industry THE
Industry Consolidation: Role of Compliance in Mergers, Acquisitions, and Divestitures Prepared for CBI s Pharmaceutical Compliance Congress April 28, 2017 M&A Activity in the Pharmaceutical Industry THE
SPECIALTY PHARMA DRIVING THE EMERGENCE OF ACCESS HUBS FOR PRIVATE PAYERS
 SPECIALTY PHARMA DRIVING THE EMERGENCE OF ACCESS HUBS FOR PRIVATE PAYERS SPECIALTY PHARMA DRIVING THE EMERGENCE OF ACCESS HUBS FOR PRIVATE PAYERS The rising cost of drugs, fuelled by the growing specialty
SPECIALTY PHARMA DRIVING THE EMERGENCE OF ACCESS HUBS FOR PRIVATE PAYERS SPECIALTY PHARMA DRIVING THE EMERGENCE OF ACCESS HUBS FOR PRIVATE PAYERS The rising cost of drugs, fuelled by the growing specialty
Product Reimbursement Services and Patient Assistance Programs KATHY CHAURETTE ALESSANDRO MARTUSCELLI
 Product Reimbursement Services and Patient Assistance Programs KATHY CHAURETTE ALESSANDRO MARTUSCELLI Overview of Legal Framework OIG Guidance Pharmaceutical manufacturers may provide certain support services
Product Reimbursement Services and Patient Assistance Programs KATHY CHAURETTE ALESSANDRO MARTUSCELLI Overview of Legal Framework OIG Guidance Pharmaceutical manufacturers may provide certain support services
Increasing pressure on PBMs to identify fraudulent providers
 Increasing pressure on PBMs to identify fraudulent providers How PBMs can use data, analytics and advanced technology to reduce their risk In July 2017, the Justice Department arrested more than 400 people
Increasing pressure on PBMs to identify fraudulent providers How PBMs can use data, analytics and advanced technology to reduce their risk In July 2017, the Justice Department arrested more than 400 people
Prevention Of Corruption
 Prevention Of Corruption Global Compliance Table Of Contents Standards Application page 6 Purpose page 5 Scope page 6 Bribery/Improper Payments, page 8 Ethical Business Practices, page 8 Unfair Business
Prevention Of Corruption Global Compliance Table Of Contents Standards Application page 6 Purpose page 5 Scope page 6 Bribery/Improper Payments, page 8 Ethical Business Practices, page 8 Unfair Business
Catalog of Services Medicare Compliance Services for Workers Compensation and Liability Claims
 Catalog of Services Medicare Compliance Services for Workers Compensation and Liability Claims With Optum, you can expect industry-leading settlement services and insight at competitive prices and, more
Catalog of Services Medicare Compliance Services for Workers Compensation and Liability Claims With Optum, you can expect industry-leading settlement services and insight at competitive prices and, more
Putting the Pieces Together, a Review of the Benefits Investigation Process. Thomas Cohn, Asembia
 Putting the Pieces Together, a Review of the Benefits Investigation Process Thomas Cohn, Asembia Introductions Thomas Cohn Chief Strategy Officer Asembia Tony Scheuth CEO and Managing Partner Point-of-Care
Putting the Pieces Together, a Review of the Benefits Investigation Process Thomas Cohn, Asembia Introductions Thomas Cohn Chief Strategy Officer Asembia Tony Scheuth CEO and Managing Partner Point-of-Care
PHARMACY GENERAL INFORMATION
 Pharmacy Program Cenpatico Integrated Care (Cenpatico IC) is committed to providing appropriate high quality and cost-effective medication therapy to all Cenpatico IC members. Cenpatico IC works with providers
Pharmacy Program Cenpatico Integrated Care (Cenpatico IC) is committed to providing appropriate high quality and cost-effective medication therapy to all Cenpatico IC members. Cenpatico IC works with providers
Pharmaceutical Management Commercial Plans
 Pharmaceutical Management Commercial Plans 2015 Toll Free Contact Number: (888) 327-0671 Medical Management: (810) 733-9711 Visit our website at: MclarenHealthPlan.org Introduction Pharmaceutical Management
Pharmaceutical Management Commercial Plans 2015 Toll Free Contact Number: (888) 327-0671 Medical Management: (810) 733-9711 Visit our website at: MclarenHealthPlan.org Introduction Pharmaceutical Management
Workers Compensation Board Pharmacy Benefit Plan
 1.0 Introduction Workers Compensation Board Pharmacy Benefit Plan Options for pharmaceutical care have greatly expanded over the past several years. New pharmaceuticals and pharmaceutical treatment modalities
1.0 Introduction Workers Compensation Board Pharmacy Benefit Plan Options for pharmaceutical care have greatly expanded over the past several years. New pharmaceuticals and pharmaceutical treatment modalities
ACTIVELY MANAGED DRUG SOLUTIONS ADVISOR. for maintenance and specialty medication. Product Guide
 ADVISOR ACTIVELY MANAGED DRUG SOLUTIONS for maintenance and specialty medication Product Guide Actively Managed Drug Solutions is not available in the province of Quebec WHAT S THE PROBLEM? Chronic disease
ADVISOR ACTIVELY MANAGED DRUG SOLUTIONS for maintenance and specialty medication Product Guide Actively Managed Drug Solutions is not available in the province of Quebec WHAT S THE PROBLEM? Chronic disease
Re: Modernizing Part D and Medicare Advantage to Lower Drug Prices and Reduce Out-of- Pocket Expenses [CMS-4180-P]
![Re: Modernizing Part D and Medicare Advantage to Lower Drug Prices and Reduce Out-of- Pocket Expenses [CMS-4180-P] Re: Modernizing Part D and Medicare Advantage to Lower Drug Prices and Reduce Out-of- Pocket Expenses [CMS-4180-P]](/thumbs/88/115848006.jpg) January 25, 2019 Seema Verma, Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-4180-P P.O. Box 8013 Baltimore, MD 21244-8013 Re: Modernizing
January 25, 2019 Seema Verma, Administrator Centers for Medicare & Medicaid Services Department of Health and Human Services Attention: CMS-4180-P P.O. Box 8013 Baltimore, MD 21244-8013 Re: Modernizing
Before prescribing ZYTIGA (abiraterone acetate), please see accompanying full Prescribing Information. Help simplify starting and staying on ZYTIGA
 Before prescribing ZYTIGA (abiraterone acetate), please see accompanying full Prescribing Information. Help simplify starting and staying on ZYTIGA Janssen CarePath helps your patients start and stay on
Before prescribing ZYTIGA (abiraterone acetate), please see accompanying full Prescribing Information. Help simplify starting and staying on ZYTIGA Janssen CarePath helps your patients start and stay on
FREQUENTLY ASKED QUESTIONS ABOUT THE CVS CAREMARK PRESCRIPTION DRUG PROGRAM
 FREQUENTLY ASKED QUESTIONS ABOUT THE CVS CAREMARK PRESCRIPTION DRUG PROGRAM ABBVIE EMPLOYEES WANT TO KNOW 2018 Pharmacy Benefit Changes Q. What is the new prior authorization program? A. Certain brand
FREQUENTLY ASKED QUESTIONS ABOUT THE CVS CAREMARK PRESCRIPTION DRUG PROGRAM ABBVIE EMPLOYEES WANT TO KNOW 2018 Pharmacy Benefit Changes Q. What is the new prior authorization program? A. Certain brand
WorldatWork You and Your PBM: Improving Discounts, Fees and Rebates, and Beyond. Kristin Begley, Pharm.D. Principal
 WorldatWork You and Your PBM: Improving Discounts, Fees and Rebates, and Beyond Kristin Begley, Pharm.D. Principal Presentation Overview The future of drug trend Prescription drug management levers: Contracting
WorldatWork You and Your PBM: Improving Discounts, Fees and Rebates, and Beyond Kristin Begley, Pharm.D. Principal Presentation Overview The future of drug trend Prescription drug management levers: Contracting
Survey Analysis of January 2014 CMS Medicare Part D Proposed Rule
 Survey Analysis of January 2014 CMS Medicare Part D Proposed Rule Prepared for: Pharmaceutical Care Management Association Prepared by: Stephen J. Kaczmarek, FSA, MAAA Principal and Consulting Actuary
Survey Analysis of January 2014 CMS Medicare Part D Proposed Rule Prepared for: Pharmaceutical Care Management Association Prepared by: Stephen J. Kaczmarek, FSA, MAAA Principal and Consulting Actuary
PRESCRIPTION DRUG SPENDING IN THE U.S. HEALTH CARE SYSTEM: AN ACTUARIAL PERSPECTIVE
 PRESCRIPTION DRUG SPENDING IN THE U.S. HEALTH CARE SYSTEM: AN ACTUARIAL PERSPECTIVE Moderator Audrey Halvorson, Vice Chairperson, Health Practice Council Presenters Karen Bender, Member, Prescription Drug
PRESCRIPTION DRUG SPENDING IN THE U.S. HEALTH CARE SYSTEM: AN ACTUARIAL PERSPECTIVE Moderator Audrey Halvorson, Vice Chairperson, Health Practice Council Presenters Karen Bender, Member, Prescription Drug
2012 Medicare Part D Transition Process for contracts H3864 & H4754:
 2012 Medicare Part D Transition Process for contracts H3864 & H4754: Essentials Rx 6, Essentials Rx 14, Essentials Rx 15, Essentials Rx 16, Premier Rx 7, Explorer Rx 1, Explorer Rx 2, and Explorer Rx 4
2012 Medicare Part D Transition Process for contracts H3864 & H4754: Essentials Rx 6, Essentials Rx 14, Essentials Rx 15, Essentials Rx 16, Premier Rx 7, Explorer Rx 1, Explorer Rx 2, and Explorer Rx 4
OHSU Center for Evidence-based Policy Rhonda Anderson, RPh Director of Pharmacy National Conference of State Legislators San Diego, CA December 10,
 OHSU Center for Evidence-based Policy Rhonda Anderson, RPh Director of Pharmacy National Conference of State Legislators San Diego, CA December 10, 2017 Today s Presentation Center for Evidence-based Policy
OHSU Center for Evidence-based Policy Rhonda Anderson, RPh Director of Pharmacy National Conference of State Legislators San Diego, CA December 10, 2017 Today s Presentation Center for Evidence-based Policy
SPECIALTY PHARMACY ACCREDITATION V3.0 MANDATORY MEASURES
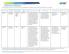 MANDATORY S Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to on an annual basis. # DESCRIPTION NUMERATOR DENOMINATOR DATA SOURCE DM2012-13 Drug-Drug
MANDATORY S Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to on an annual basis. # DESCRIPTION NUMERATOR DENOMINATOR DATA SOURCE DM2012-13 Drug-Drug
Florida Medicaid Prescribed Drug Service Spending Control Initiatives. For the Quarter April 1, 2016 through June 30, 2016
 Florida Medicaid Prescribed Drug Service Spending Control Initiatives For the Quarter April 1, through June 30, Report to the Florida Legislature December 2017 [This page intentionally left blank.] Table
Florida Medicaid Prescribed Drug Service Spending Control Initiatives For the Quarter April 1, through June 30, Report to the Florida Legislature December 2017 [This page intentionally left blank.] Table
Florida Medicaid Prescribed Drug Service Spending Control Initiatives. For the Quarter July 1, 2016 through September 30, 2016
 Florida Medicaid Prescribed Drug Service Spending Control Initiatives For the Quarter July 1, through September 30, Report to the Florida Legislature March 2018 [This page intentionally left blank.] Table
Florida Medicaid Prescribed Drug Service Spending Control Initiatives For the Quarter July 1, through September 30, Report to the Florida Legislature March 2018 [This page intentionally left blank.] Table
Value Propositions in Contractual Relationships:
 Value Propositions in Contractual Relationships: Real World Evidence, Outcomes Research, and Comparative Effectiveness Presented by: October 22, 2015 BJ D'Avella Senior Director, Huron Life Sciences, New
Value Propositions in Contractual Relationships: Real World Evidence, Outcomes Research, and Comparative Effectiveness Presented by: October 22, 2015 BJ D'Avella Senior Director, Huron Life Sciences, New
OHSU Center for Evidence-based Policy Rhonda Anderson, RPh Director of Pharmacy EMPAA 2017 October 30, 2017
 OHSU Center for Evidence-based Policy Rhonda Anderson, RPh Director of Pharmacy EMPAA 2017 October 30, 2017 Wedding Day Preparation The Big Moment is Here Mr. & Mrs. Anderson Today s Presentation Center
OHSU Center for Evidence-based Policy Rhonda Anderson, RPh Director of Pharmacy EMPAA 2017 October 30, 2017 Wedding Day Preparation The Big Moment is Here Mr. & Mrs. Anderson Today s Presentation Center
Re: Medicare Prescription Drug Benefit Manual Draft Chapter 5
 September 18, 2006 BY ELECTRONIC DELIVERY Cynthia Tudor, Ph.D. Director, Medicare Drug Benefit Group Centers for Medicare and Medicaid Services Department of Health and Human Services Mail Stop C4-13-01
September 18, 2006 BY ELECTRONIC DELIVERY Cynthia Tudor, Ph.D. Director, Medicare Drug Benefit Group Centers for Medicare and Medicaid Services Department of Health and Human Services Mail Stop C4-13-01
Medicare Part D Transition Policy
 Medicare Part D Transition Policy Transition Policy for New and Current Enrollees of our Medicare Part D Prescription Drug Plan PURPOSE: Simply Healthcare Plans, Inc. must maintain an appropriate transition
Medicare Part D Transition Policy Transition Policy for New and Current Enrollees of our Medicare Part D Prescription Drug Plan PURPOSE: Simply Healthcare Plans, Inc. must maintain an appropriate transition
Pharmacy Benefit Strategies for Lowering Prescription Drug Costs
 Pharmacy Benefit Strategies for Lowering Prescription Drug Costs Mid-sized Retirement and Healthcare Plan Management Conference San Francisco, CA March 17, 2014 Pharmacy Benefit Strategies for Lowering
Pharmacy Benefit Strategies for Lowering Prescription Drug Costs Mid-sized Retirement and Healthcare Plan Management Conference San Francisco, CA March 17, 2014 Pharmacy Benefit Strategies for Lowering
SETTING A STANDARD FOR GP COMPLIANCE
 SETTING A STANDARD FOR GP COMPLIANCE CURRENT LANDSCAPE AND WHAT DOES GP COMPLIANCE LOOK LIKE? MAY 9, 2017 2017 HURON CONSULTING GROUP INC. SPEAKER INTRODUCTIONS Clay Willis Director T 404-825-3319 E cwillis@huronconsultinggroup.com
SETTING A STANDARD FOR GP COMPLIANCE CURRENT LANDSCAPE AND WHAT DOES GP COMPLIANCE LOOK LIKE? MAY 9, 2017 2017 HURON CONSULTING GROUP INC. SPEAKER INTRODUCTIONS Clay Willis Director T 404-825-3319 E cwillis@huronconsultinggroup.com
The Health Plan has processes in place that explain how members, pharmacists, and physicians:
 Introduction Overview The Health Plan shall promote optimal therapeutic use of pharmaceuticals by encouraging the use of cost effective generic and/or brand drugs in certain therapeutic classes. The Health
Introduction Overview The Health Plan shall promote optimal therapeutic use of pharmaceuticals by encouraging the use of cost effective generic and/or brand drugs in certain therapeutic classes. The Health
Key Medicare Issues for Coverage and Reimbursement of Specialty Pharmaceuticals
 Key Medicare Issues for Coverage and Reimbursement of Specialty Pharmaceuticals By Cindy Parks Thomas, Ph.D. A dvances in biotechnology have brought many effective new treatments for serious and debilitating
Key Medicare Issues for Coverage and Reimbursement of Specialty Pharmaceuticals By Cindy Parks Thomas, Ph.D. A dvances in biotechnology have brought many effective new treatments for serious and debilitating
2015 PacificSource Medicare Part D Transition Process for contracts H3864 & H4754:
 2015 PacificSource Medicare Part D Transition Process for contracts H3864 & H4754: Essentials Rx 6 (HMO), Essentials Rx 14 (HMO), Essentials Rx 15 (HMO), Essentials Rx 16 (HMO), Essentials Rx 19 (HMO),
2015 PacificSource Medicare Part D Transition Process for contracts H3864 & H4754: Essentials Rx 6 (HMO), Essentials Rx 14 (HMO), Essentials Rx 15 (HMO), Essentials Rx 16 (HMO), Essentials Rx 19 (HMO),
Overview of Reimbursement Strategies for Novel Medical Technologies
 Overview of Reimbursement Strategies for Novel Medical Technologies Nov 9, 2016 Goals and Objectives Develop understanding of U.S. medical technology reimbursement landscape and provide information about
Overview of Reimbursement Strategies for Novel Medical Technologies Nov 9, 2016 Goals and Objectives Develop understanding of U.S. medical technology reimbursement landscape and provide information about
Chapter 8 Section 9.1
 Other Services Chapter 8 Section 9.1 Issue Date: August 2002 Authority: 32 CFR 199.2(b), 32 CFR 199.4(b)(2)(vi), (b)(3)(iii), (b)(5)(v), (d)(3)(vi), (e)(11)(i), 32 CFR 199.5(d)(12); 32 CFR 199.17, and
Other Services Chapter 8 Section 9.1 Issue Date: August 2002 Authority: 32 CFR 199.2(b), 32 CFR 199.4(b)(2)(vi), (b)(3)(iii), (b)(5)(v), (d)(3)(vi), (e)(11)(i), 32 CFR 199.5(d)(12); 32 CFR 199.17, and
Insert Slide Title. Jennifer A. Romanski, Esq. February 8, 2017
 Insert Slide Title Jennifer A. Romanski, Esq. February 8, 2017 Interactions/Engagements with Healthcare Professionals (HCPs) and Healthcare Organizations (HCOs) Consulting Payments Service Arrangements
Insert Slide Title Jennifer A. Romanski, Esq. February 8, 2017 Interactions/Engagements with Healthcare Professionals (HCPs) and Healthcare Organizations (HCOs) Consulting Payments Service Arrangements
Supplemental Special Advisory Bulletin: Independent Charity. Patients who cannot afford their cost-sharing obligations
 Supplemental Special Advisory Bulletin: Independent Charity Patient Assistance Programs I. Introduction Patients who cannot afford their cost-sharing obligations for prescription drugs may be able to obtain
Supplemental Special Advisory Bulletin: Independent Charity Patient Assistance Programs I. Introduction Patients who cannot afford their cost-sharing obligations for prescription drugs may be able to obtain
Developments in Recent Corporate Integrity Agreements (CIAs)
 Developments in Recent Corporate Integrity Agreements (CIAs) Jonathan Levy PDMA Alliance Board Member Summer 2015 The following has been prepared by The PDMA Alliance for use by its Members as educational
Developments in Recent Corporate Integrity Agreements (CIAs) Jonathan Levy PDMA Alliance Board Member Summer 2015 The following has been prepared by The PDMA Alliance for use by its Members as educational
DEFICIT REDUCTION ACT AND FALSE CLAIMS POLICY INFORMATION FOR All MASSACHUSETTS WORKFORCE MEMBERS
 DEFICIT REDUCTION ACT AND FALSE CLAIMS POLICY INFORMATION FOR All MASSACHUSETTS WORKFORCE MEMBERS The Company is committed to preventing health care fraud, waste and abuse and complying with applicable
DEFICIT REDUCTION ACT AND FALSE CLAIMS POLICY INFORMATION FOR All MASSACHUSETTS WORKFORCE MEMBERS The Company is committed to preventing health care fraud, waste and abuse and complying with applicable
PI Compensation: Methods, Documentation, and Execution
 PI Compensation: Methods, Documentation, and Execution David B. Russell, CRCP Director, Site Strategy Liz Christianson Client engagement manager PFS CLINICAL 2018 PharmaSeek Financial Services, LLC d.b.a.
PI Compensation: Methods, Documentation, and Execution David B. Russell, CRCP Director, Site Strategy Liz Christianson Client engagement manager PFS CLINICAL 2018 PharmaSeek Financial Services, LLC d.b.a.
PI Compensation: Methods, Documentation, and Execution
 PI Compensation: Methods, Documentation, and Execution David B. Russell, CRCP Director, Site Strategy Liz Christianson Client engagement manager PFS CLINICAL 2018 PharmaSeek Financial Services, LLC d.b.a.
PI Compensation: Methods, Documentation, and Execution David B. Russell, CRCP Director, Site Strategy Liz Christianson Client engagement manager PFS CLINICAL 2018 PharmaSeek Financial Services, LLC d.b.a.
PHARMACY COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 1/18/18 SECTION: DRUGS LAST REVIEW DATE: 8/13/18 LAST CRITERIA REVISION DATE: ARCHIVE DATE:
 STEP THERAPY Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage Guideline must
STEP THERAPY Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage Guideline must
Current Issues in Patient and Product Support. October 20, 2016
 Current Issues in Patient and Product Support October 20, 2016 How Did a Perennial Issue Become the Hot Topic? 1. Reimbursement Support 2. Patient Assistance Programs 3. Donations to Charitable Foundations
Current Issues in Patient and Product Support October 20, 2016 How Did a Perennial Issue Become the Hot Topic? 1. Reimbursement Support 2. Patient Assistance Programs 3. Donations to Charitable Foundations
SPECIALTY PHARMACY MANDATORY MEASURES
 SPECIALTY PHARMACY MANDATORY S Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis. # DESCRIPTION NUMERATOR DENOMINATOR DTM
SPECIALTY PHARMACY MANDATORY S Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis. # DESCRIPTION NUMERATOR DENOMINATOR DTM
Specialty Pharmacy Trends: Payer and Industry Considerations for Specialty Pharmacies
 Specialty Pharmacy Trends: Payer and Industry Considerations for Specialty Pharmacies September 18, 2017 Washington, DC Frier & Levitt, LLC Jonathan E. Levitt, JD Co-Founding Partner jlevitt@frierlevitt.com
Specialty Pharmacy Trends: Payer and Industry Considerations for Specialty Pharmacies September 18, 2017 Washington, DC Frier & Levitt, LLC Jonathan E. Levitt, JD Co-Founding Partner jlevitt@frierlevitt.com
Specialty Pharmacy + Medication Assistance Programs
 Specialty Pharmacy + Medication Assistance Programs Presenters: Scott Sterrett, PharmD Manager, Specialty Pharmacy Beaumont Health FACULTY DISCLOSURE The faculty reported the following financial relationships
Specialty Pharmacy + Medication Assistance Programs Presenters: Scott Sterrett, PharmD Manager, Specialty Pharmacy Beaumont Health FACULTY DISCLOSURE The faculty reported the following financial relationships
THIRD-PARTY PHARMACY RECONCILIATION
 THIRD-PARTY PHARMACY RECONCILIATION Billy Caster Sales Solution Expert Inmar Healthcare Network Jon Brumbaugh Sr. Manager, Product Inmar Healthcare Network Session Description A discussion and presentation
THIRD-PARTY PHARMACY RECONCILIATION Billy Caster Sales Solution Expert Inmar Healthcare Network Jon Brumbaugh Sr. Manager, Product Inmar Healthcare Network Session Description A discussion and presentation
DEFICIT REDUCTION ACT AND FALSE CLAIMS POLICY INFORMATION FOR All NEW YORK WORKFORCE MEMBERS
 DEFICIT REDUCTION ACT AND FALSE CLAIMS POLICY INFORMATION FOR All NEW YORK WORKFORCE MEMBERS The Company is committed to preventing health care fraud, waste and abuse and complying with applicable state
DEFICIT REDUCTION ACT AND FALSE CLAIMS POLICY INFORMATION FOR All NEW YORK WORKFORCE MEMBERS The Company is committed to preventing health care fraud, waste and abuse and complying with applicable state
2019 Pre-Medicare Retiree Healthcare Open Enrollment
 2019 Pre-Medicare Retiree Healthcare Open Enrollment CHANGES ONLY ENROLLMENT Submit Enrollment Changes Before November 21 You MUST complete and submit the enclosed enrollment form by November 21 if you
2019 Pre-Medicare Retiree Healthcare Open Enrollment CHANGES ONLY ENROLLMENT Submit Enrollment Changes Before November 21 You MUST complete and submit the enclosed enrollment form by November 21 if you
MemberChoice FORMULARY MANAGEMENT MEDICATION THERAPY MANAGEMENT (MTM) SPECIALTY DRUG MANAGEMENT. Specialty Drug Management
 MemberChoice FORMULARY MANAGEMENT MEDICATION THERAPY MANAGEMENT (MTM) SPECIALTY DRUG MANAGEMENT SPECIALTY DRUG MANAGEMENT 1 1% Prescriptions Written in 2012 99% 25% Prescription Drug Spending in 2012 75%
MemberChoice FORMULARY MANAGEMENT MEDICATION THERAPY MANAGEMENT (MTM) SPECIALTY DRUG MANAGEMENT SPECIALTY DRUG MANAGEMENT 1 1% Prescriptions Written in 2012 99% 25% Prescription Drug Spending in 2012 75%
Florida Medicaid Prescribed Drug Service Spending Control Initiatives
 Florida Medicaid Prescribed Drug Service Spending Control Initiatives For the Quarters January 1, through March 31, and April 1, through June 30, Report to the Florida Legislature April 2018 [This page
Florida Medicaid Prescribed Drug Service Spending Control Initiatives For the Quarters January 1, through March 31, and April 1, through June 30, Report to the Florida Legislature April 2018 [This page
The Transition to Value-Based Health Care: Recommendations for Medical Device Manufacturers
 The Transition to Value-Based Health Care: Recommendations for Medical Device Manufacturers April 27, 2017 LLP Agenda Introduction Shift to Value-Based Care New Models of Medical Device Company Operation
The Transition to Value-Based Health Care: Recommendations for Medical Device Manufacturers April 27, 2017 LLP Agenda Introduction Shift to Value-Based Care New Models of Medical Device Company Operation
Pharmaceutical Compliance Congress: State of the States
 Pharmaceutical Compliance Congress: State of the States October 27, 2008 Janice G. Cunningham Jeffrey L. Handwerker Overview Types of State Laws Potentially Affected by the Sunshine Act Limits or Prohibitions
Pharmaceutical Compliance Congress: State of the States October 27, 2008 Janice G. Cunningham Jeffrey L. Handwerker Overview Types of State Laws Potentially Affected by the Sunshine Act Limits or Prohibitions
PhRMA Perspective: Government Policies to Support Innovative Contracting Approaches
 PhRMA Perspective: Government Policies to Support Innovative Contracting Approaches CBI s PAP 2017 Michelle Drozd, Deputy Vice President Policy & Research Department October 12, 2016 Agenda Recent trends
PhRMA Perspective: Government Policies to Support Innovative Contracting Approaches CBI s PAP 2017 Michelle Drozd, Deputy Vice President Policy & Research Department October 12, 2016 Agenda Recent trends
Individual Business Prescription Drug Utilization Management Changes Frequently Asked Questions
 Individual Business Prescription Drug Utilization Management Changes Frequently Asked Questions Overview: Up to six prescription drug utilization/benefit management (UM) programs will be added to the individual
Individual Business Prescription Drug Utilization Management Changes Frequently Asked Questions Overview: Up to six prescription drug utilization/benefit management (UM) programs will be added to the individual
Introducing. Manulife DrugWatch. Applying rigorous oversight to help ensure value for money in a dramatically changing drug market
 Introducing Manulife DrugWatch Applying rigorous oversight to help ensure value for money in a dramatically changing drug market The drug market in Canada is changing rapidly and dramatically Many Canadians
Introducing Manulife DrugWatch Applying rigorous oversight to help ensure value for money in a dramatically changing drug market The drug market in Canada is changing rapidly and dramatically Many Canadians
2019 Pre-Medicare Retiree Healthcare Open Enrollment
 2019 Pre-Medicare Retiree Healthcare Open Enrollment CHANGES ONLY ENROLLMENT Submit Enrollment Changes Before November 21 You MUST complete and submit the enclosed enrollment form by November 21 if you
2019 Pre-Medicare Retiree Healthcare Open Enrollment CHANGES ONLY ENROLLMENT Submit Enrollment Changes Before November 21 You MUST complete and submit the enclosed enrollment form by November 21 if you
Risk Contracting: What to Know About Stop Loss Insurance KATHRYN A BOWEN, EXECUTIVE VICE-PRESIDENT OCTOBER 27, 2016
 Risk Contracting: What to Know About Stop Loss Insurance KATHRYN A BOWEN, EXECUTIVE VICE-PRESIDENT OCTOBER 27, 2016 Provider Stop Loss Insurance Premiums Program Structure Losses within Retention What
Risk Contracting: What to Know About Stop Loss Insurance KATHRYN A BOWEN, EXECUTIVE VICE-PRESIDENT OCTOBER 27, 2016 Provider Stop Loss Insurance Premiums Program Structure Losses within Retention What
